Abstract
Abstract 4451
Bosutinib, a dual Src/Abl tyrosine kinase inhibitor (TKI) has demonstrated efficacy in a phase I/II study of patients with Imatinib-Resistant or Imatinib-Intolerant Chronic Phase Chronic Myeloid Leukemia (CP CML). Bosutinib was associated with an acceptable safety profile and mild to moderate diarrhea was the most common non-hematologic toxicity. Since diarrhea is a common side effect of TKI therapy, an exploratory objective of the phase I/II trial was to observe how bothersome this side effect was to patient perceived health-related quality of life.
The Functional Assessment of Cancer Therapy- Leukemia (FACT-Leu) was used to evaluate patient reported health-related quality of life (HRQoL) at baseline, prior to treatment, and at weeks 4, 8, 12, every 12-weeks thereafter, and at treatment completion. The modular FACT-Leu uses a 7-day recall period and consists of a core set of general cancer questions and a leukemia specific subscale with 5 domains: Social Well-being (SWB), Emotional Well-being (EWB), Physical Well-being (PWB), Functional Well-being (FWB) and Leukemia Subscale (LeuS). Select questions from the PWB (“I am bothered by side effects of treatment”) and FWB (“I am content with the quality of my life right now”) were used to describe how bothersome diarrhea was to patient HRQoL. To evaluate how bothersome diarrhea was to the patient and how content patients were with their quality of life, a 5-point Likert response format was used (0, not at all; 1, a little bit; 2, somewhat; 3, quite a bit; and 4, very much). Patients' who experienced diarrhea within the 7-days prior to completion of the HRQoL instrument were classified according to diarrhea grade (CTCAE v3.0) and patients not experiencing diarrhea within the recall period were classified as grade 0 (Table 1).
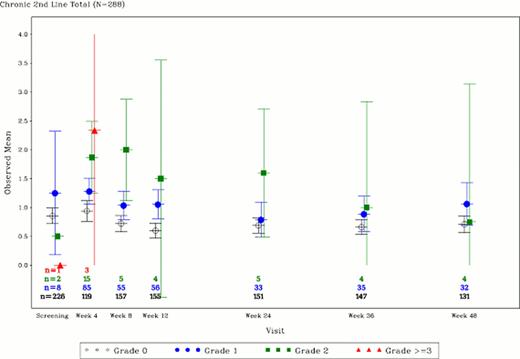
Of the N=288 CP CML patients treated with bosutinib following imatinib failure (second-line TKI) included in the trial, n=237 (82%) completed the bothered by side effects (BSE) question at baseline and n=249 (86%) completed the content with quality of life (CQoL) at baseline. The observed mean scores on the BSE question by diarrhea grade are presented in Figure 1. For each time point, patients not experiencing diarrhea (grade 0) or those experiencing grade 1 diarrhea reported being bothered by the side effects of their treatment “a little bit” (range = 0.6 – 1.3) and patients experiencing grade 2 diarrhea reported being “a little bit” to “somewhat” bothered by the side effects of their treatment (range = 0.5 – 2.0). The mean score on the bothersome side effect between groups for each week was statistically significant at weeks 4, 8, 12, and 24 (p<0.05); these analysis were not adjusted for multiple comparisons. Mean CQoL scores for patients with grade 1 diarrhea were: baseline (2.5), wk4 (2.5), wk8 (2.5), wk12 (2.6), wk24 (2.9), wk36 (2.7), and wk48 (2.7) indicating patients were “somewhat” content with the quality of their life. Similarly, mean CQoL scores for patients with grade 2 diarrhea were: baseline (2.3), wk4 (2.5), wk8 (2.0), wk12 (3.0), wk24 (2.2), wk36 (2.8), and wk48 (2.0) indicating patients were “somewhat” content with the quality of their life.
Patients treated with Bosutinib for Imatinib-Resistant or Imatinib-Intolerant CP CML did not report diarrhea to be a bothersome side effect of therapy and patients were content with their quality of life. These results highlight the value of capturing patient perceived HRQoL to better understand the impact of treatment.
FACT-Leu Observed Mean Scores for Bothered by Side Effects of Treatment
Whiteley:Pfizer Inc: Employment, Equity Ownership. Reisman:Pfizer Inc: Employment, Equity Ownership. Kelly:Pfizer Inc: Employment, Equity Ownership. Cortes:Novartis, Bristol Myers Squibb, Pfizer, Ariad, Chemgenex: Consultancy, Research Funding. Cella:Pfizer Inc: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal