Abstract
Abstract 4448
Bosutinib is a dual Src/Abl tyrosine kinase inhibitor (TKI), which has demonstrated efficacy in a phase I/II study of patients with Advanced Phase Chronic Myeloid Leukemia (CML). The objective was to evaluate the effect of bosutinib on health -related quality of life (HRQoL) in patients with advanced CML after failure with imatinib.
Patient reported HRQoL was an exploratory objective in the clinical trial and measured using the 44-item Functional Assessment of Cancer Therapy-Leukemia (FACT-Leu). The FACT-Leu is a modular approach to assess patient HRQoL using a core set of general cancer questions as well as a cancer site specific leukemia subscale with 5 domains: Social Well-being (SWB), Emotional Well-being (EWB), Physical Well-being (PWB), Functional Well-being (FWB) and Leukemia Subscale (LeuS); and 3 summary scales: FACT-General, FACT Trial Outcome Index (TOI) and FACT-Leu Total. The item responses for each scale are summed to provide scores; higher scores indicate better HRQoL. The FACT-Leu was completed at weeks 4, 8, 12 and every 12 weeks thereafter, as well as treatment completion. Within cohort comparisons were assessed using paired t-tests.
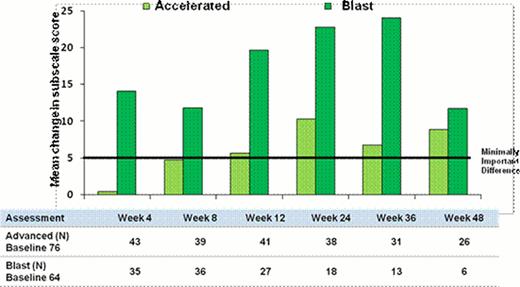
Of the 164 patients with advanced leukemia included in the trial, 76 had accelerated phase (AP) CML and 64 blast phase (BP) CML. At 24 weeks, AP patients reported statistically significant improvements in PWB (p=0.02), EWB (p<0.001), LeuS (p<0.001), FACT-G (p<0.001), FACT-Leu (p<0.001) and FACT-TOI (p<0.001) with the PWB, FACT-G, LeuS, TOI and FACT-Leu exceeding minimally important differences (MID) at 24 weeks. Blast phase patients reported significant improvements in PWB (p=0.02), EWB (p=0.02), FWB (p=0.04), LeuS (p=0.01), FACT-G (p<0.001), FACT-Leu (p<0.001) and FACT-TOI (p=0.01) at 24-weeks with all scales exceeding MID except the SWB. At 48-weeks the AP patients continued to have statistically significant improvements in PWB (p=0.05), EWB (p=0.02), and FACT-G (p=0.03) and PWB, FACT-G, TOI and FACT-Leu exceeded the MID at 48 weeks. There were no statistically significant deteriorations in HRQoL through week 48 (Figure 1)
These data suggest that CML patients treated with bosutinib demonstrate improved HRQoL. Confirmation in a controlled study is needed.
Whiteley:Pfizer Inc: Employment, Equity Ownership. Reisman:Pfizer Inc: Employment, Equity Ownership. Kelly:Pfizer Inc: Employment, Equity Ownership. Cortes:Novartis, Bristol Myers Squibb, Pfizer, Ariad, Chemgenex: Consultancy, Research Funding. Cella:Pfizer Inc: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal