Abstract
Abstract 4409
PBPC mobilization with intermediate-dose cyclophosphamide (3–4 gm/m2) (ID-CY) and G-CSF when compared to low-dose CY (1–2 gm/m2)-based strategies, has been shown to have a favorable risk/benefit profile in MM patients (pts) receiving novel induction therapies (Hamadani M. BBMT 2012). However the relative efficacy of ID-CY as compared to plerixafor in PBPC mobilization in MM pts is not known. Herein we report outcomes of ID-CY/G-CSF mobilization compared to plerixafor/G-CSF mobilization in MM pts treated with novel induction regimens.
This multicenter outcomes study includes 84 pts who underwent a planned, single autograft within 1-year of starting induction therapy with novel chemotherapy agents (thalidomide, lenalidomide, bortezomib) from 2003–2012. Consecutive pts undergoing mobilization with ID-CY/G-CSF (3–4 gm/m2) (n=55) at one institution were compared against consecutive pts receiving plerixafor/G-CSF (0.24 mg/kg) (n=29) at a different transplant center. At baseline the two groups were compared for parameters predicting mobilization failure. In order to assess efficiency of PBPC mobilization, we evaluated peak peripheral blood (PB) CD34+ cell counts, CD34+ cell yield on day1 of collection, total CD34+ cell collection, and total number of apheresis sessions. Mobilization failure was defined as failure to collect ≥2 ×106 cells/kg body weight. All pts with normal renal function received uniform conditioning with Mel200 (MEL140 if serum creatinine was ≥2 mg/dl).
At baseline, the ID-CY and plerixafor cohorts were well balanced (Table 1). No difference was observed in the use of lenalidomide (p=0.3). Compared to plerixafor, ID-CY use was associated with higher median peak PB CD34+ cell count (63/μl vs. 160/μl, p=0.01), CD34+ yield on day 1 of collection (6.5 ×106/kg vs. 11.7 ×106/kg, p=0.004), and total CD34+ cell yield (10.5 ×106/kg vs. 24.9 ×106/kg, p=0.001). Median numbers of apheresis sessions were 2.2 in each group (p=0.9). No mobilization failures were seen in either group. There was no difference in the proportion of pts collecting ≥5×106/kg CD34+ cells in either group (93% vs. 96%, p=0.6), but more pts in ID-CY cohort collected ≥10×106/kg CD34+ cells (55% vs. 78%, p=0.02). Neutrophil engraftment was significantly faster (9.9 days vs. 13.1 days, p<0.001) in the ID-CY pts, likely because of higher infused CD34+ cell dose. Rate of adverse events were higher in the ID-CY cohort including neutropenic fevers (p=0.02), intravenous antibiotic use (p=0.03), hospitalization (p=0.05) and packed red cell transfusions (p=0.007).
In the era of novel agents compared to plerixafor, ID-CY produced a more robust PBPC mobilization, faster engraftment, but was associated with significantly higher (but manageable) toxicity, and no difference in mobilization failure rates. These data support use of either ID-CY or plerixafor-based PBPC mobilization in MM pts undergoing stem cell collection following novel induction therapies.
Baseline characteristics.
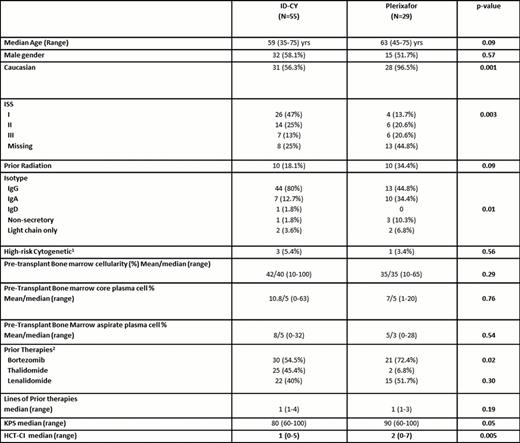
1Defined as hypodiploidy or deletion 13 on conventional cytogenetics or presence of t(4;14), t(14:16), -17p on FISH and/or conventional cytogenetics.
2Percentages do not add up 100% since some pts received more than 1 novel agent either in combination or during different lines of therapy.
Awan:Allos Therapeutics: Speakers Bureau. Hamadani:Conquer Cancer Foundation of ASCO: Research Funding; ASBMT & Millennium New Investigator Award: Research Funding; American Cancer Society 116837-IRG-09–061-01: Research Funding; Celgene Corp: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal