Abstract
Abstract 4358
Warfarin is an oral vitamin K antagonist commonly used in pediatric patients who require anticoagulation. Studies in adults receiving warfarin demonstrate that polymorphisms in 2 genes involved in warfarin disposition and response, cytochrome P450 2C9 (CYP2C9) and vitamin K epoxide reductase complex, subunit 1 (VKORC1), explain 30–40% of the variation in stable dose. Recent evidence suggests a role for a cytochrome P450 4F2 (CYP4F2) variant in altered warfarin disposition. These associations have not been well-studied in the pediatric population. We hypothesized that polymorphic variants in CYP2C9, VKORC1, and CYP4F2 are associated with interindividual variability in stable warfarin dose in pediatric patients.
We designed a cross-sectional study examining the relationship between stable warfarin dose and commonly occurring polymorphisms in CYP2C9 (*2 and *3), VKORC1 (haplotype A, comprised of 5 promoter and intronic variants), and CYP4F2 (variant allele T) in children. Patients <25 years old who had begun, received, or completed warfarin therapy at <20 years were eligible for recruitment. Patients were identified in 2 ways: a search of our institutional electronic medical record (StarPanel) or a search of the Synthetic Derivative (SD), the deidentified electronic medical record which correlates to the Vanderbilt BioVU DNA biorepository, a DNA databank with >16,000 pediatric DNA samples.
Patients identified from StarPanel were approached at a Vanderbilt clinic appointment or by mail/phone call for enrollment. Data was collected via study questionnaire and review of StarPanel, including demographic factors and covariates such as concomitant medications, co-morbidities, warfarin indication, and diet history. Warfarin dose and INR history were also collected. The same information, excluding study questionnaire data, was collected from the SD for the BioVU population. The BioVU and StarPanel populations were cross-referenced to prevent inclusion of duplicate patients.
Genomic DNA was extracted from peripheral blood lymphocytes obtained from blood drawn with routine labs or from epithelial cells from a saliva sample. All samples were genotyped for CYP2C9, VKORC1, and CYP4F2 variants using a validated Taqman-based PCR method.
One-hundred seventeen patients (45 StarPanel, 72 BioVU) with mean age 12.5 years at time of stable warfarin dose were recruited and met eligibility criteria for inclusion. 55.6% of the patients were female and the majority (82.9%) were Caucasian, though African-Americans (10.3%) and other minorities were also represented. The majority of patients had DVT (47%) or prosthetic valve (26.5%) as indications for warfarin therapy. There was a statistically significant correlation between height and stable warfarin dose (r=-0.346, p<0.0001).
Sixteen patients (13.6%) had bleeding complications while on warfarin, 8 of whom required hospitalization. No bleeding event resulted in death or long-term sequelae. Two patients had more than 1 bleeding event reported. Four patients (3.4%) had thrombosis while on warfarin. Two of the 4 received no additional therapy and had complete resolution of symptoms. No thrombotic event resulted in death.
Genotype analysis for CYP2C9, VKORC1, and CYP4F2 variants has been completed in the StarPanel population, and genotyping in the BioVU population is currently being completed. To date, variant allele frequencies are similar to published population frequencies, with 38.6% VKORC1 haplotype A, 10.5% CYP2C9*2, 4.7% CYP2C9*3, and 32.3% CYP4F2 haplotype T.
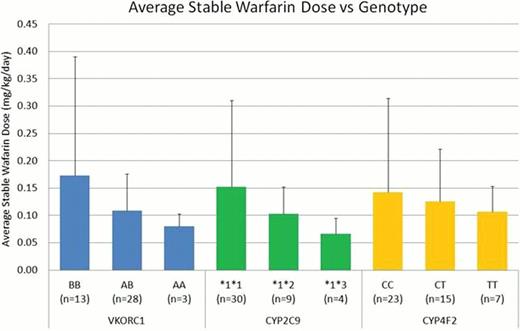
Preliminary analysis supports previously published data indicating a role for genotype in warfarin sensitivity in children (Figure 1). Patients homozygous for the VKORC1 A haplotype tended to require a lower warfarin dose to achieve target INR. Patients with variant CYP alleles (CYP2C9*2, CYP2C9*3, or CYP4F2 variant T) tended to require lower warfarin doses to achieve target INR. VKORC1 appeared to demonstrate a gene dosage effect with a lower dose requirement for each variant allele present. Our results suggest polymorphisms in VKORC1 and CYP2C9 contribute to altered warfarin disposition and response in pediatric patients.
Preliminary comparison of stable warfarin dose and genotype in the StarPanel population
Preliminary comparison of stable warfarin dose and genotype in the StarPanel population
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal