Abstract
Abstract  435
435
Early results for the randomized phase-III CLL8 trial of the German CLL study group (GCLLSG) showed that the addition of a monoclonal antibody, rituximab (R), to a first-line chemotherapy composed of fludarabine and cyclophosphamide (FC) improved both the progression-free survival (PFS) and the overall survival (OS) of patients with CLL (Hallek M and Fischer K et al., Lancet 2010). On the basis of these findings FCR has become the standard therapy for physically fit patients with previously untreated CLL. In this report, we present the results of an extended follow up of the CLL8 trial, with particular emphasis on survival data and late adverse events such as secondary malignancies and late neutropenia following chemoimmunotherapy with FCR.
In this prospective, randomized, open-label phase-III trial, 817 treatment-na•ve patients with good physical fitness and CD20-positive CLL received six courses of either FCR or FC. The primary endpoint of the study was progression-free survival (PFS). Secondary endpoints were safety, response to treatment and overall survival (OS).
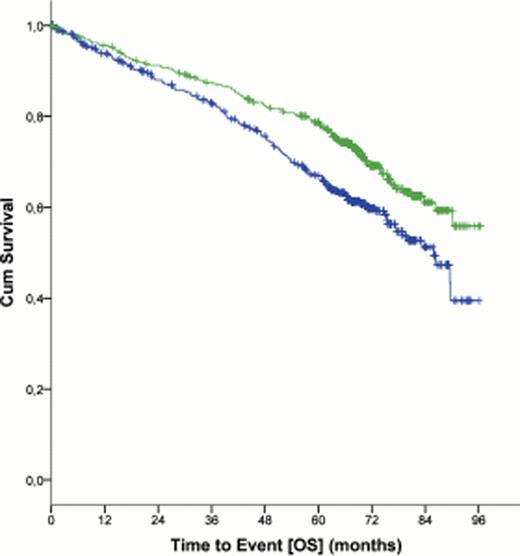
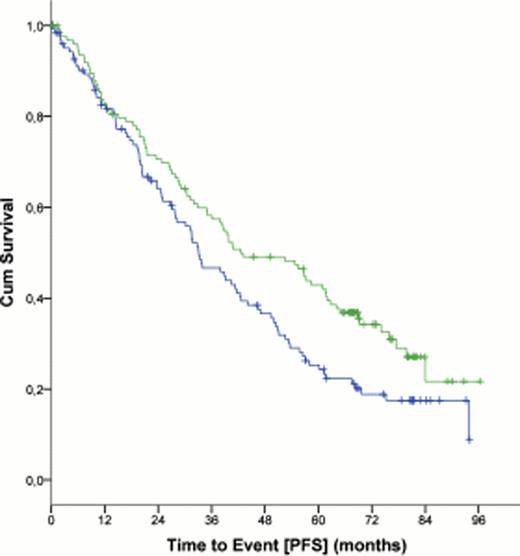
At a median follow up of 5.9 years, 38.0% of the patients in the FCR group were free of disease progression compared with 27.4% in the FC group (hazard ratio, 0.6 (CI 95%, 0.5 – 0.7), p<0.0001). At the same time-point, 69.4% of the patients were alive in the FCR group versus 62.3% in the FC group (hazard ratio 0.7 (95% CI, 0.5 – 0.9), p=0.001; figure 1). Median OS was not reached for patients in the FCR group, while median OS was 86.0 months (95% CI, 78.7 – 93.2 months) for the FC group (p = 0.001). Significant improvements in PFS were achieved in all Binet stages including patients with Binet stage C disease (median PFS, 42.5 months for patients in the FCR group versus 33.1 months for patients in the FC group (hazard ratio, 0.7 (95% CI, 0.5 – 0.9), p = 0.02; figure 2).
All patients who had received at least one of the study drugs were included in the safety analysis (n=800). The frequency of secondary malignancies with a mean time to onset of 2.4 years after the end of treatment (range 2.0 days – 7.4 years) was independent of the treatment given (40 patients (9.9%) in the FCR group versus 48 patients (12.1%) in the FC group (p = 0.4)). Of these 88 patients, 46 patients (5.7%) had a solid tumor, 33 patients (4.1%) had a Richter transformation, and 12 patients (1.5%) had a myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML). These secondary malignancies did not occur with any difference when comparing the FCR with the FC arm. Late neutropenias as defined by the presence of grade 3 or 4 neutropenia 2 months after end of treatment, were noted more frequently in the FCR group (67 patients, 16.6%) compared to the FC group (35 patients, 8.8%) (p=0.007)). Late neutropenias occurred predominantly during the first year after end of FCR treatment and decreased one year after end of treatment (16 patients (3.9%) in the FCR group versus 15 patients (3.7%) in the FC group, p=0.7).
The results of an extended follow up of the CLL8 protocol of the GCLLSG corroborate the concept of a superior outcome for patients that received FCR chemoimmunotherapy. Secondary malignancies occurred at 9.9% in the FCR arm and at 12.1% in the FC arm. FCR induced an increase in prolonged neutropenias during the first year after end of treatment but not thereafter. These prolonged neutropenias following FCR did not translate into an increased rate of MDS/AML thus far.
Progression Free Survival (PFS) in patients with Binet stage C disease (n = 252)
Progression Free Survival (PFS) in patients with Binet stage C disease (n = 252)
Fischer:Roche: Honoraria, travel grants Other. Bahlo:Roche: Honoraria. Fink:Roche: travel grants Other. Böttcher:Roche: Honoraria, Research Funding. Mayer:Roche: Consultancy, Research Funding. Kneba:Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding. Eichhorst:Roche: Honoraria, Research Funding. Wendtner:Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Stilgenbauer:Roche: Consultancy, Honoraria, Research Funding. Hallek:Roche: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal