Abstract
Abstract 4341
Acute myeloid leukemia (AML) incidence increases with age, yet treatment of elderly patients has reduced efficacy compared with younger patients and is often poorly tolerated. We aimed to determine the outcome of elderly patients with de novo AML treated with intensive chemotherapy with or without allogeneic stem cell transplantation.
All patients with de novo AML (excluding APML) aged ≥ 60 years treated with induction chemotherapy at our institutions between February 1999 and July 2011 were retrospectively identified from institutional databases. Information on cytogenetic risk, chemotherapy protocols, response to therapy, disease-free survival (DFS), and overall survival (OS) were then determined retrospectively by review of individual medical records. Survival analyses were calculated by the Kaplan-Meier method and compared using the log-rank test.
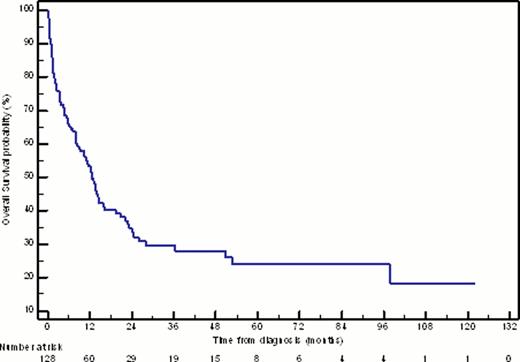
Intensive induction chemotherapy was received by 128 patients (57.7% of elderly patients diagnosed with de novo AML), including 105 patients (82.0%) treated with standard-dose cytarabine (SDAC) (100mg/m2 days 1–7) and 14 patients (10.9%) treated with high-dose cytarabine (HiDAC) (≥ 2000mg/m2/day for at least 4 days). The median age of this cohort was 67 years (range 60–83 years). Based on cytogenetic profile, 3.1% of patients had favourable, 54.7% had intermediate, and 27.3% had adverse-risk disease. Responses to 1–2 cycles of induction chemotherapy were complete remission (CR1) in 73.4% of patients, refractory disease in 14.8%, and induction death in 11.7%. 83.0% of patients who achieved CR1 received consolidation chemotherapy, incorporating SDAC in 74.4% and HiDAC in 23.1%. At a median follow-up of 22 months for survivors, intensive induction chemotherapy resulted in a median DFS of 11 months, and median OS of 13 months (Figure 1); 3 year OS for the entire cohort was 27.9%, with favourable, intermediate, and adverse risk groups having 50.0%, 31.6%, and 12.6% 3 year OS, respectively.
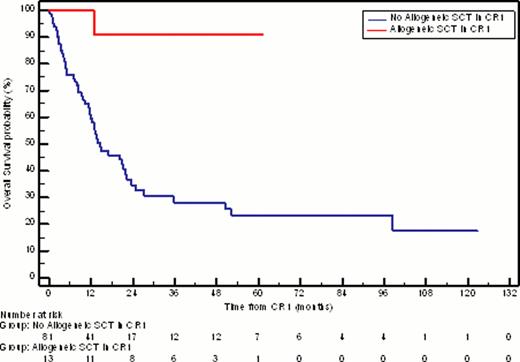
Thirteen patients (10.2%) proceeded to allogeneic transplantation in CR1; median age was 63 years (range 60–66 years). These patients did not reach median DFS or OS; 11 (84.6%) remain alive and disease-free at a median follow-up of 27 months post-transplant (Figure 2).
The elderly patients were compared to the cohort of younger patients (15–59 years) with de novo AML treated over the same time period. This analysis found that the older patients had a higher rate of adverse-risk cytogenetics (27.3% vs 16.1%, respectively; P = 0.02), refractory disease (14.8% vs 3.3%, respectively; P = 0.0002) and induction death (11.7% vs 4.7%, respectively; P = 0.03), and lower CR1 rate (73.4% vs 91.9%, respectively; P < 0.0001), DFS (median DFS 11 months vs 25 months, respectively; P = 0.0009) and OS (median OS 13 months vs 78 months, respectively; P < 0.0001).
Despite intensive chemotherapy, the majority of patients ≥ 60 years with AML have poor outcomes, with high rates of induction death, refractory disease, and relapsed AML. However, a proportion of these patients experience long-term survival. While patients selected for allogeneic transplantation in CR1 have high DFS and OS, only a minority of patients receive this therapy.
Kaplan-Meier estimate of overall survival for patients ≥ 60 years treated with intensive induction chemotherapy for newly-diagnosed AML.
Kaplan-Meier estimate of overall survival for patients ≥ 60 years treated with intensive induction chemotherapy for newly-diagnosed AML.
Kaplan-Meier estimate of overall survival for patients in CR1 who received allogeneic stem cell transplantation compared with those who did not (median OS = NR vs 14.6 months, respectively; P = 0.0009).
Kaplan-Meier estimate of overall survival for patients in CR1 who received allogeneic stem cell transplantation compared with those who did not (median OS = NR vs 14.6 months, respectively; P = 0.0009).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal