Abstract
Abstract 4314
The prognosis for patients with primary induction failure (PIF) or relapsed acute myeloid leukemia (rel-AML) is poor. Dose intense etoposide (E) and cyclophosphamide (C) used alone or in combination therapy, without stem cell transplantation (HSCT) have been successfully used as salvage therapy for hematological malignancies. Difference in chemotherapy dose and schedule has been a limitation in the evaluation of previous E and C studies. Additionally, previous EC studies were conducted before karyotype was found to be a major determinant of prognosis. Currently the influence of karyotype on response to dose-intense EC is unknown. We report the response to treatment with dose intense EC combination therapy in 34 heavily pretreated PIF or rel-AML patients, most with intermediate or unfavorable cytogenetics.
34 consecutive patients with PIF or Rel-AML treated with dose-intense EC between 10/2004-12/2011 were evaluated. All patients received initial induction therapy with daunorubicin 45–90mg/m2 IV bolus d1–3 and cytarabine 100mg/m2 CI d1–7, and consolidation with HIDAC for 1–3 courses if CR was achieved. Treatment: 27 patients (79%) received etoposide 3gm/m2 by continuous infusion over 48–72hours followed by cyclophosphamide 50mg/kg iv infusion daily for 3 or 4 days. The remaining 7 patients received reduced etoposide 1.8–2.4gm/m2. The decision to use a given dose of EC was left to the discretion of the treating physician. Chi-Square, Fischers Exact Test and Cox regression analysis were bused for statistical analysis.
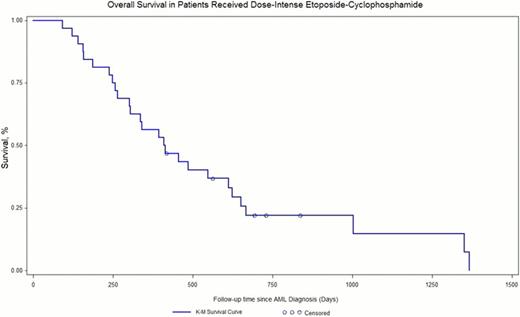
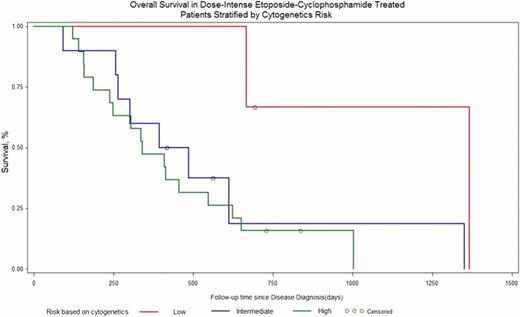
Patient demographics can be seen in Table 1. Most patients had rel-AML (62%), previous exposure to high or intermediate-dose cytarabine (75%), were < 60 years old (70%), had intermediate or unfavorable cytogenetics (92%), and received at least 1 salvage regimen (in addition to induction and consolidation) prior to dose-intense EC (65%). Patient outcomes are seen in Table 2. Overall, CR was achieved in 32% of patients. Median duration of CR after DI-EC treatment was 5 months. Figure 1 shows overall survival curve. No significant difference in response was found for patient's age >60years (p<0.3085), gender (p<0.1345), exposure to high or intermediate dose cytarabine (p<0.4254), the number of salvage regimens received prior to EP (p<0.1672), or etoposide dose intensity (p<0.5172). Rel-AML patients had significantly higher CR rates compared to PIF (p<0.0145). Subset analysis shows unfavorable karyotype (Figure 2) to be associated with a poorer response (p<0.0223). Grade 3–4 GI toxicity was observed in 19(59%) patients, which was not associated with etoposide dose level (p<0.99) Microbiologically confirmed infections occurred in 26(76%) patients but was not associated with etoposide dose. Treatment related mortality was observed in 6(18%) patients.
Dose intense EP therapy was moderately effective in achieving CR, especially in heavily pre-treated rel-AML patients. The regimen was successful as a temporizing treatment prior to allogeneic HSCT. Infection rates are high and warrant close surveillance and early aggressive antibiotic therapy.
Demographics
| Characteristic . | Number . |
|---|---|
| Age (range) | 48 (25–70) |
| Age>60 (percent) | 10 (29) |
| Male(%) | 14 (41) |
| Indication | |
| Relapsed-AML | 21 (62) |
| Primary refractory AML | 13 (38) |
| Initial CR duration (months) | 8 (2–72) |
| Karotype | |
| Good | 3 (9) |
| Intermediate | 12 (35) |
| Poor | 19 (56) |
| HSCT prior to Herzig | 5 (15) |
| Herzig prior to HSCT | 12 (35) |
| Prior exposure to HIDAC | 25 (75) |
| Number of salvage regimens prior to Herzig | |
| n=0 | 12 |
| n=1 | 13 |
| n>1 | 9 |
| Dose Levels | |
| E(1.8-2.4g/m2) and CY (150-200mg/m2) | 7 (34) |
| E(3g/m2) and CY (150-200mg/m2) | 27 (66) |
| Results | |
| Overall CR (percent) | 11/34 (32) |
| CR duration (months) | 5 |
| Response (%) | |
| Primary Refractory AML | 1/13 (8) |
| Relapsed AML | 10/21 (48) |
| Response by dose level (%) | |
| EP (1.8-2.4gm/m2) | 1/7 (14) |
| EP (3gm/m2) | 10/27 (37) |
| Response by karyotype (%) | |
| Favorable | 3/3 (100) |
| Intermediate | 2/12 (17) |
| Poor | 6/19 (32) |
| Response by age (%) | |
| <60 | 3/10 (30) |
| >60 | 8/24 (33) |
| Response by the number prior salvage treatments (%) | |
| 0 | 3/12 (25) |
| 1 | 5/13 (38) |
| 2 | 1/6 (17) |
| 3 | 2/2 (100) |
| Grade 3-4 GI toxicity (%) | 19/34 (59) |
| Microbiologically confirmed infection (%) | 26 (76) |
| Treatment related mortality | 6/34 (18) |
| Characteristic . | Number . |
|---|---|
| Age (range) | 48 (25–70) |
| Age>60 (percent) | 10 (29) |
| Male(%) | 14 (41) |
| Indication | |
| Relapsed-AML | 21 (62) |
| Primary refractory AML | 13 (38) |
| Initial CR duration (months) | 8 (2–72) |
| Karotype | |
| Good | 3 (9) |
| Intermediate | 12 (35) |
| Poor | 19 (56) |
| HSCT prior to Herzig | 5 (15) |
| Herzig prior to HSCT | 12 (35) |
| Prior exposure to HIDAC | 25 (75) |
| Number of salvage regimens prior to Herzig | |
| n=0 | 12 |
| n=1 | 13 |
| n>1 | 9 |
| Dose Levels | |
| E(1.8-2.4g/m2) and CY (150-200mg/m2) | 7 (34) |
| E(3g/m2) and CY (150-200mg/m2) | 27 (66) |
| Results | |
| Overall CR (percent) | 11/34 (32) |
| CR duration (months) | 5 |
| Response (%) | |
| Primary Refractory AML | 1/13 (8) |
| Relapsed AML | 10/21 (48) |
| Response by dose level (%) | |
| EP (1.8-2.4gm/m2) | 1/7 (14) |
| EP (3gm/m2) | 10/27 (37) |
| Response by karyotype (%) | |
| Favorable | 3/3 (100) |
| Intermediate | 2/12 (17) |
| Poor | 6/19 (32) |
| Response by age (%) | |
| <60 | 3/10 (30) |
| >60 | 8/24 (33) |
| Response by the number prior salvage treatments (%) | |
| 0 | 3/12 (25) |
| 1 | 5/13 (38) |
| 2 | 1/6 (17) |
| 3 | 2/2 (100) |
| Grade 3-4 GI toxicity (%) | 19/34 (59) |
| Microbiologically confirmed infection (%) | 26 (76) |
| Treatment related mortality | 6/34 (18) |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal