Abstract
Abstract 4205
Relapse is the primary cause of treatment failure in patients who undergo alloHCT for hematologic malignancies. We describe the presentation, management, and outcomes of children who relapse post-HCT to improve monitoring and intervention strategies.
This was a single institution, retrospective cohort study of children with relapse or progression of acute lymphoblastic leukemia (ALL), acute myelogenous leukemia (AML), mixed phenotypic acute leukemia (MPAL) or myelodysplastic syndrome (MDS) post-alloHCT between January 1, 2003 and December 31, 2010. Relapse was defined as any evidence for disease detected by any modality (including morphologic, cytogenetic, flow cytometric, molecular and radiographic methods) after previously negative results. Progressive disease was defined as an increase in any measure from baseline results. Minimal residual disease (MRD) was defined as disease detectable by immunophenotypic, cytogenetic or molecular methods that did not meet classic morphologic criteria for relapse (defined as > 5% disease).
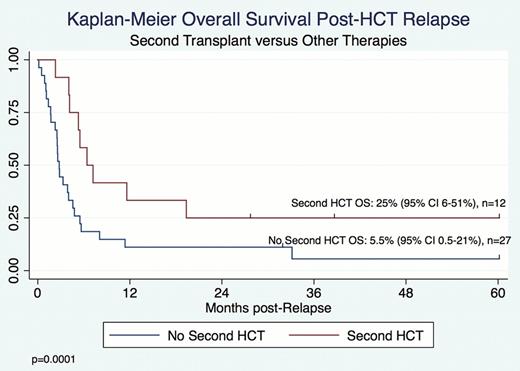
40 patients with AML (n=18), ALL (n=16), MPAL (n=4) and MDS (n=2) were identified who relapsed from among 93 patients who underwent a first alloHCT. The median time from alloHCT to relapse was 144 days (range 1 day– 58 months). Presentation of relapse included: detection of asymptomatic disease found on scheduled restaging (n=17); presentation with peripheral blasts or overt extramedullary disease (n=17); and presentation with symptoms prompting evaluation for recurrent disease, including hematologic abnormalities and pain (n=5). Two patients had isolated, asymptomatic central nervous system relapse. Salvage therapies included: supportive care, including hospice, palliative or alternative care only, (n=6); withdrawal of immunosuppression (WIS) (n=3); cytoreductive and/or radiation therapy with or without additional therapy (n=24), donor lymphocyte infusion (DLI) with or without prior chemotherapy (n=13) and second HCT (n=12). Nine patients with only MRD at the time of relapse presented at a median time of 35 days post-HCT (range 28–182 days). Although these patients were immediately considered candidates for intervention and despite 4 patients being treated with WIS, most had very rapid progression of disease with evidence for frank relapse at a median time of 23 days post-initial MRD detection. 2 patients died from transplant-related-mortality shortly after detection of MRD. Median survival after relapse was 123 days (range 4 days–5 years). Estimated 6-month and 1-year post-relapse survival were 30% and 17.5%, respectively. Five of 40 (12.5%) patients are currently alive with a median follow-up of 39 months (range 1 day–58 months), including 1 patient with active disease. 1 survivor had MDS and presented with MRD alone. The remaining 4 presented with overt disease and included 3 patients with ALL and 1 with MPAL. 3 of 5 survivors underwent a second HCT. Overall survival (OS) was superior in patients who underwent a second HCT (p=0.0001), with a 25% OS after second HCT (Figure 1). No patients with AML survived post-relapse even though 6/18 underwent second HCT. There was no survival advantage for those whose relapse was detected as MRD as compared to overt disease. Long-term survivors include 3 patients with overt relapse between 146 and 411 days post-HCT.
40 of 93 (43%) patients with hematologic malignancies relapsed after alloHCT. After relapse, 1-year OS was 17.5% with a median survival of 124 days post-relapse. Although pre-emptive treatment of relapse in the setting of MRD is felt to be ideal, it may not be feasible. In our study, patients presenting with MRD only presented very early post-HCT (median time 35 days) at a time when post-HCT complications can be high and limit therapeutic options. Additionally, once MRD was detected, disease progression to frank relapse was rapid (median time 23 days) limiting the chance to respond to many frontline immunotherapeutic options such as WIS or DLI. Given the poor outcomes of relapse post-HCT and limited ability to treat relapse even when detected at the stage of MRD, efforts should focus on developing more effective therapies and preventing relapse by identifying those at highest risk of relapse as candidates for novel methods to enhance efficacy of alloHCT.
OS by Second HCT for Patients with Post-alloHCT Relapse
OS by Second HCT for Patients with Post-alloHCT Relapse
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal