Abstract
Abstract 4160
There is limited data on the prevalence, clinical profile and outcome of patients with β thalassemia major (TM) who is HCV sero-positive (HCV+) prior to an allogeneic stem cell transplant (SCT). From October, 1991 to June, 2012, 370 SCT were done for TM at our center. The median age was 7.5 years (range: 2–24) and there were 232 (62.87%) males. 209 (56.5%) belonged to Lucarelli Class III. There were 44 (11.9%) cases who were HCV+, 6 (1.6%) who were HBsAg positive and 350 (94.6%) who were CMV sero-positive at the time of pre-transplant screening.
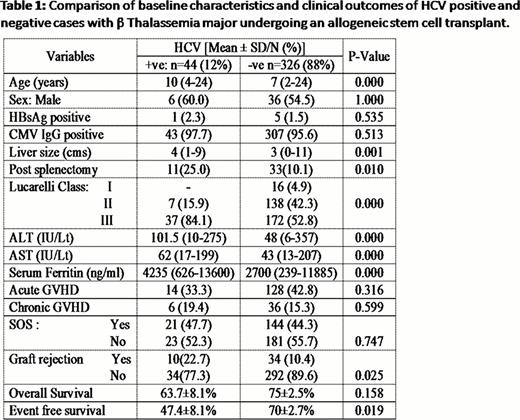
HCV+ cases were significantly older and had a significantly larger liver size. There were significantly more number of HCV+ cases who were Lucarelli Class III and the median liver enzyme and ferritin levels were higher in the HCV+ group. Table 1 summarizes these and other major differences between those who were HCV positive at diagnosis and the remaining patients. The number of graft rejections was significantly higher in the HCV+ group while the TRM was comparable between the two groups. This translated into a significantly inferior event free survival (P=0.016) though the overall survival was not significantly different (P=0.140)(Table 1). It is well recognized that patients with TM are at a high risk of developing sinusoidal obstruction syndrome (SOS) post SCT. In this series, 165 (4.6%) patients developed SOS. HCV+ cases did not have a significantly higher incidence of SOS compared to those that were HCV negative. In only 3 (6.8%) of HCV+ cases was SOS the major cause of death while SOS was a major contributory factor for death in 17 (5.2%) of the remaining cases. Among the 15 (34%) of HCV+ cases that died, the major contributory cause of death was infection (40%), graft failure (20%) and SOS (20%). On a univariate cox regression analysis, HCV+ had an adverse impact on EFS (RR=1.710; 95%CI 1.066–1.744; P=0.026). However, on a forward stepwise multivariate analysis after adjusting for conventional risk factors HCV+ status did not confer an independent adverse risk factor. Of the 29 patients who are surviving, at a median follow up of 25 months, none have had progression to chronic liver disease. 20 (69%) had a normal liver function test at last follow up while only 3 have had HCV directed therapy post transplant.
From August, 2009 at our center we have been using a treosulfan based conditioning regimen for our Class III patients. Among the HCV+ cases 10 were conditioned with this regimen. This regimen was well tolerated with 100% engraftment. Two patients died, one due to sepsis and the other to GVHD. In comparison, in the historical control of HCV+ cases conditioned with a conventional busulfan based regimen where there were graft rejections in 9 (26.4%) and 13 (38.2%) deaths, the major contributory cause for death being infections (46%), SOS (23%) and graft failure (23%).
In conclusion HCV+ status is a surrogate risk factor for other adverse risk factors such as older age, increased liver size and inadequate medical therapy prior to SCT. After adjusting for such risk factors HCV+ cases with TM undergoing an allogeneic SCT transplant have comparable outcomes to HCV negative cases. Use of a treosulfan based regimen is well tolerated in the HCV+ group and could potentially improve the outcome in this high risk group.
Comparison of baseline characteristics and clinicical outcomes of HCV positive and vegative cases with β thalassemia major undergoing an allogeneic stem cell transplant.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal