Abstract
Abstract 4157
We previously reported promising results with nonmyeloablative (NMA) allogeneic transplantation for indolent non-Hodgkin's lymphomas (NHL) (Khouri et al, Blood 2012; 119:6373). This strategy is however less effective in aggressive, bulky, chemoresistant, or heavily pre-treated lymphomas. The BEAM-rituximab is a commonly used regimen in transplantation for NHL. Bortezomib has synergistic activity with rituximab, is effective against many NHL subtypes with moderate non-hematologic toxicity; in addition, animal models suggest that pre-transplant bortezomib may reduce the risk of GVHD. We hypothesized that the addition of bortezomib to the conditioning would enhance initial disease control and that remission could be later sustained via the GVL effect of the graft, with lesser GVHD.
We included pts up to 70 yrs, with relapsed/refractory NHL, acceptable organ function, having a matched donor, and who were not eligible for NMA transplantation. Bortezomib was designed to be given iv in an escalated dose of 1.0, 1.3, and 1.6 mg/m2 days-13, -6, - 1 and + 2. Rituximab was given at a dose of 375 mg/m2 on day -13 and 1000 mg/m2 on days -6, +1, and +8, as previously described. BEAM consisted of carmustine 300 mg/m2day-6, cytarabine 100 mg/m2 and etoposide 100 mg/m2 twice/day, days -5 to -2, and melphalan100 mg/m2 on day-1. Tacrolimus and methotrexate was used for GVHD prophylaxis. In addition, thymoglobulin of 1 mg/kg was given on days -2, and -1 in pts receiving an unrelated donor.
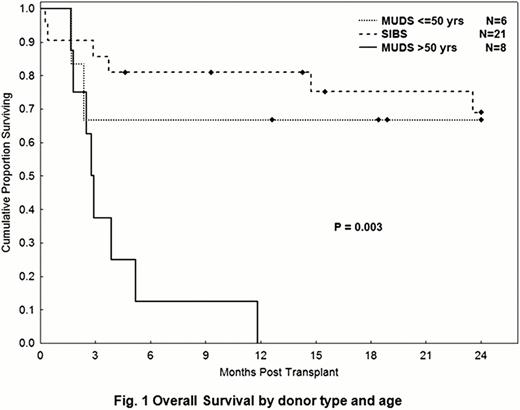
The study included 35 pts [Aggressive DLBCL=20, Indolent (CLL/FL with aggressive features =15)]. All pts had relapsed disease after the best conventional therapy (-ies) available. Median age was 53 (range, 22–65) years. Median prior treatments were 3 (range, 1–7). At transplant, 14 pts (51%) had refractory disease and 23 (66%) were PET+. Twenty-two pts (63%) received their transplants from HLA-compatible siblings (Sibs) and 13 (37%) from unrelated donors (MUDs). Bortezomib could not be escalated beyond the initial 1.0 mg/m2 per dose due to toxicity (2 of 3 pts treated at 1.3 mg/m2 died of sepsis related to C.Difficile; since then all pts received flagyl prophylaxis till engraftment). Blood was the source of donor graft in 97% of pts. All pts engrafted donor cells. Median donor T cells at day 30 were 100%. With a median follow-up time of 24.5 months (range, 5–49), the OS for the aggressive and indolent histologies were 58% and 44%, respectively (p=0.5); PFS rates were 32% in both. Determinants of outcomes were related to donor type, and age. In pts <50 yrs, the 2-yrs OS rates for Sibs and MUDs were 76% and 67%, respectively (p=0.4) (Fig 1). In pts >50 yrs, the 2-years OS and PFS for sibs were 62% and 45%, respectively, vs 0% for the MUDs (Fig 1). There was no difference in pts and disease characteristics, or histology distribution between the 4 groups. In pts < 50 yrs, 2-year treatment-related mortality in Sibs and MUDs was 22% and 17% (p=0.9); whereas the incidence was significantly higher in >50 yrs pts receiving MUDs (62% vs 37%, p=0.03). This difference may be related to a higher incidence in acute 2–4 GVHD in >50 yrs pts receiving MUDs transplants (62% vs 33% in pts >50yrs receiving Sibs transplants). In <50 yrs pts, the incidence of acute 2–4 GVHD in the Sibs and MUDs transplants were 44% and 33%, respectively (p=0.8). Eighteen pts died: causes were related to infection [(n=6, (2 of which in the setting of GVHD)], acute GVHD (n=3), chronic GVHD (n=4), progression (n=3) and unknown (n=2).
Our results suggest that in pts with high-risk NHL and who were not eligible for NMA allogeneic transplantation, the bortezomib-BEAM-rituximab conditioning has promising PFS rates of 40% at 2-years in pts younger than 50 yrs. Alternative strategies are needed for pts >50 yrs, receiving an unrelated donor transplant. Results warrant further study and refinement.
Khouri:Millenium Pharmaceuticals: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal