Abstract
Abstract 4096
Despite aggressive hematopoietic cell transplant (HCT) strategies, many patients with acute myeloid leukemia (AML) relapse. Our group has explored radioimmunotherapy (RIT) using anti-CD45 antibodies (Ab) labeled with β-emitting radionuclides such as 131I and 90Y as a means to augment cytotoxicity and reduce relapse. This approach has been limited by their low energy levels (0.66–2.3 MeV) and potential non-specific toxicities due to their relatively long path lengths (0.3–2.3 mm). Conversely, α-emitting agents display higher energy levels (8 MeV) delivered over a short path-length (∼60–80 μm) that can lead to superior therapeutic ratios of absorbed radiation doses that may reduce AML relapse. For this purpose 211At is an α-emitter with an attractive half-life (7.2 hours), energy profile (6.8 MeV averages of two alpha decays, 5.9 and 7.5 MeV) and path length (average range 55–70 μm). Therefore, we evaluated the efficacy and toxicity of anti-CD45 RIT using 211At in a clinically relevant CD45+disseminated murine leukemia model.
SJL/J mice were given 105 syngeneic SJL leukemic peripheral blood mononuclear cells (PBMCs) via tail vein, followed two days later by211At-labeled anti-murine CD45 Ab-decaborate(2-) conjugate (30F11-B10) or 211At-labeled negative control Ab-decaborate(2-) conjugate (rat IgG-B10) in tissue biodistribution studies. Groups of 5 mice were euthanized 6, 24 and 48 hours later and organs were harvested and analyzed in a gamma counter to yield percent of the injected dose per gram (% ID/g). To assess toxicities associated with this approach, SJL/J mice were treated with 12–24 μCi of 211At-30F11-B10 and then evaluated weekly thereafter for impact on blood counts, as well as changes in hepatic and renal function. In HCT therapeutic studies, groups of 10 leukemic mice per dose were injected with 12–24 μCi of 211At-30F11-B10 or 211At-rat Ab-B10, followed two days later by rescue with 15 × 106 syngeneic bone marrow (BM) cells.
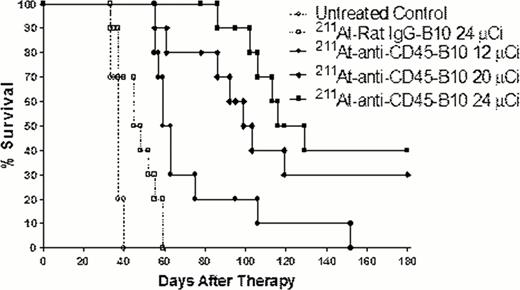
Delivery of 211At-30F1-B10 demonstrated excellent localization to the BM and spleen at 24 hours (79 and 18% ID/g, respectively) post injection with lower kidney and lung uptake (8.4 and 8.3% ID/g, respectively) at the same time point. Anti-CD45 RIT using 211At-30F11-B10 followed by syngeneic HCT led to a dose-dependent survival benefit in leukemic mice with a median survival (OS) of 120, 98 days, and 62 days for animals treated with 24, 20, and 12 μCi 211At-30F11-B10, respectively, compared with untreated control mice (median OS of 36 days) and mice treated with non-specific 211At-labeled rat Ab-B10 (median OS of 46 days) (Figure). Moreover, anti-CD45 RIT with 211At-30F11-B10 led to minimal toxicity with mild dose dependent leukopenia as the most pronounced lab abnormality. White blood cell count nadir was between 2.5 and 4.2 k/μL two weeks after HCT for mice treated with 24 and 12 μCi 211At-30F11-B10, respectively. Counts recovered to normal levels (6–8 K/μL) 4 weeks after HCT. Mild increases in transaminase levels were seen in mice that received 211At-30F11-B10, yet these values remained within the normal ranges (ALT 68–75 IU/L; AST 155–170 IU/L). Renal function after 211At-30F11-B10 did not significantly deviate from baseline (BUN 15–17 mg/dL; Cr 0.3–0.4 mg/dL).
Taken together, these data suggest that anti-CD45 RIT using the α-emitting radionuclide 211At in conjunction with HCT is a promising therapeutic option for AML. Excellent targeting of radiation doses to BM and spleen was demonstrated with a favorable toxicity profile. Further investigation of anti-CD45 RIT for AML using 211At in clinical trials appears to be warranted.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal