Abstract
Abstract 4049
Bortezomib became funded for frontline therapy of symptomatic multiple myeloma (SMM) in New Zealand on 1 May 2011. From this date all new patients requiring treatment at our institution, both transplant eligible (TE) and ineligible (TIn), were initially treated with CyBorD consisting of weekly Cyclophosphamide 300mg/m2 PO, Bortezomib 1.6mg/m2 SC(2.5mg/ml) and Dexamethasone 40mg PO. One cycle was arbitrarily defined as 28 days, TE patients received 4–6 cycles CyBorD before ABMT (using 200mg/m2 melphalan), then 3 cycles of VTD (Bortezomib 1.6mg/m2 weekly, Thalidomide 100mg daily, Dexamethasone 40mg weekly) as post transplant consolidation. TIn patients received 4 cycles of CyBorD then 4 cycles of VTD. In June 2011 we changed all patients to subcutaneous bortezomib due to ease of administration and reduced toxicity.
The records of all untreated SMM patients initiating treatment with Bortezomib at our institution from 1 May 2011 were analyzed for response.
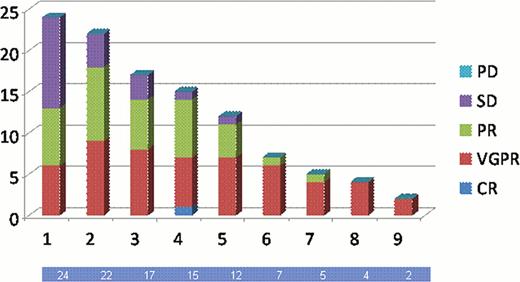
We have treated 25 patients with SMM, 14 TE and 11 TIn. The median age was 68 yrs (range 45 to 88 yrs). The treatments were well tolerated. There was one Bortezomib dose reduction due to toxicity (burning feet on VTD). Injection site skin redness was common, but local reactions were not severe, one patient changed to IV Bortezomib because of this. There were no dose delays due to thrombocytopenia, and no patient had a pre-dose thrombocytopenia of <75 × 109/l. So far 6 patients have completed transplant, no patients have failed to proceed to transplant. There have been no deaths. Responses have improved with each cycle of treatment, after the first cycle the overall response (OR) was 54% (≥VGPR 25%), after 5 cycles the OR rate was 92% (≥VGPR 58%). confirmed CR rates are low, in part as patients are awaiting final bone marrow biopsy.
Weekly subcutaneous Bortezomib is well tolerated, and has high patient acceptance. Dose limiting neuropathy is rare, and responses are high when used in combination with CyBorD or VTD.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal