Abstract
Abstract 4007
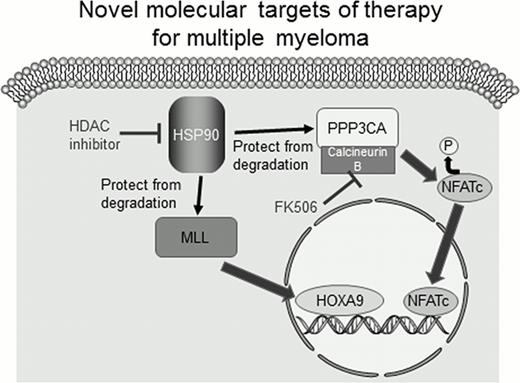
Multiple myeloma is one of incurable hematological malignancies and many novel drugs including histone deacetylase (HDAC) inhibitors are currently undergoing preclinical and clinical evaluation. Treatment of U266 and KMS-11, multiple myeloma cell lines, by LBH589, an HDAC inhibitor, inhibited proliferation and induced apoptosis of these cell lines at low nanomolar concentrations. Here, we discovered that expression of HOXA9, a homeobox protein, was suppressed in the presence of LBH589. Transcription of HOXA9 mRNA immediately decreased in LBH589-treated myeloma cell lines. HOXA9 is a candidate oncogene in multiple myeloma and knockdown of HOXA9 is shown to block proliferation of myeloma cell lines. Our results suggest that HOXA9 is one of the targets of anti-myeloma effects elicited by HDAC inhibitors. MLL (mixed-lineage leukemia), a trithorax group protein, is shown to be essential for persistent expression of HOXA9 in human leukemia cells. We examined the effect of LBH589 on MLL in myeloma cell lines and found that expression of MLL protein was suppressed without decrease of MLL mRNA. These results indicate that LBH589 induces degradation of MLL protein. In the previous studies, it was shown that HDAC inhibitors targeted heat shock protein 90 (HSP90). The molecular chaperone HSP90 is essential for the protein-folding ability of several proteins. We evaluated the expression of MLL in myeloma cell lines treated by HSP90 inhibitor. We found that the expression of MLL proteins was suppressed by the treatment of 17-AAG, an inhibitor of HSP90. These results suggest that HDAC inhibitors induce degradation of MLL proteins via inhibition of chaperone function of HSP90. This inhibition of MLL-HOXA9 by HDAC inhibitors are supposed to block proliferation of myeloma cells.
Furthermore, we tried to find a cooperative factor of MLL to investigate the roles of MLL in pathophysiology of multiple myeloma. For this purpose, we picked up PPP3CA, catalytic subunit of calcineurin, as one of the molecules. Those were highly co-expressed with MLL in multiple myeloma patients. We revealed that PPP3CA was degraded by the treatment of LBH 589 or 17-AAG. These results suggest that PPP3CA is protected from protein degradation by HSP90 as in the case of MLL and that LBH589 induces degradation of PPP3CA through inhibition of chaperone function of HSP90. We also found that co-treatment of myeloma cell lines by LBH589 and FK506 showed more anti-proliferative effect than LBH589 alone. FK506 selectively inhibits the function of calcineurin B, regulatory subunit of calcineurin. Expression of PPP3CB, the other isozyme of catalytic subunit of calcineurin, was upregulated when myeloma cell lines were treated with LBH589 and this upregulation of PPP3CB was supposed to be the result of compensation for downregulation of PPP3CA. It is suggested that combination of FK506 with LBH589 should display enhanced anti-myeloma effects by inhibiting the interaction between upregulated PPP3CB and calcineurin B. These results indicate that calcineurin-signaling pathway plays an important role in the persistent proliferation of myeloma cells. Surprisingly, bortezomib also suppressed expression of PPP3CA via inhibition of HDAC6.
Our study is the first report to demonstrate that MLL-HOXA9 and calcineurin are the important targets of HDAC inhibitors in the treatment for multiple myeloma. MLL and PPP3CA, catalytic subunit of calcineurin, are highly co-expressed in multiple myeloma patients. These results suggest that HDAC inhibitors including LBH589 display anti-myeloma effects by inhibiting both MLL-HOXA9 and calcineurin pathways. These findings will lead to understanding of a novel mechanism of survival and growth of myeloma cells and be helpful to establish a new strategy of therapy for multiple myeloma.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal