Abstract
Abstract 3913
The possibility of measuring the circulating free light chains (FLC) improved our ability to detect the amyloidogenic clone, allowed refined risk stratification in combination with cardiac biomarkers, and provided a formidable tool for assessing response to treatment in AL amyloidosis. Recently, a novel method for FLC quantitation based on monoclonal antibodies has been developed. We evaluated its performance in the diagnosis, prognostication of survival, and response assessment in 353 consecutive newly diagnosed patients with AL amyloidosis enrolled between 2007 and 2011.
Serum and urine immunofixation electrophoresis (IFE) was performed with a Hydragel 2IF/BJ(HR) kit on a Hydrasis apparatus (Sebia). Serum FLC concentration was measured in duplicate on frozen sera by a polyclonal (Binding Site) and a monoclonal (Siemens) immunoassay on a Behring BNII nephelometer. Reference ranges are k-FLC 3.3–19.4 mg/L, l-FLC 5.7–26.3 mg/L, k/l ratio 0.26–1.65 for the Binding Site (BS) assay, and k-FLC 6.7–22.4 mg/L, l-FLC 8.3–27.0 mg/L, k/l ratio 0.31–1.56 for the Siemens (S) assay. Response was evaluated 3 months after treatment initiation.
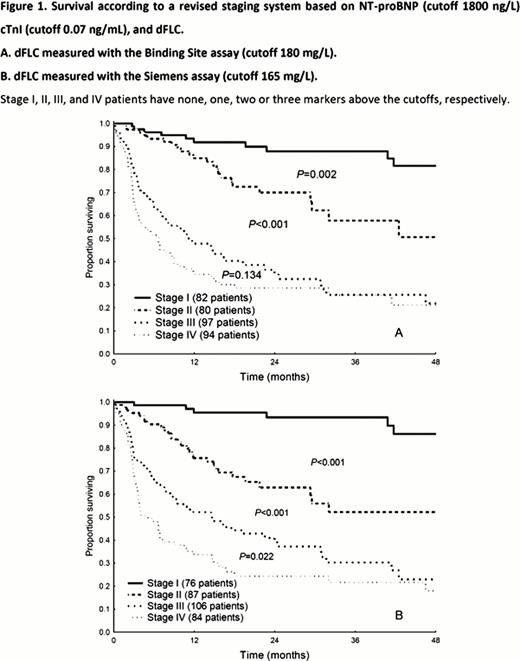
The deposited amyloidogenic light chain identified by immuno electron microscopy was k in 69 patients (19%) and l in 284 (81%). Fifteen patients (4%) were excluded from the calculation of the diagnostic sensitivity because a biclonal gammapathy was detected by IFE. The two FLC assays had similar diagnostic sensitivity (Table 1). We calculated the concordance correlation coefficient to evaluate the agreement between the two assays, which was better for k (0.92, 95%CI 0.87–0.91) than for l (0.78, 95%CI 0.73–0.82) FLC. A total of 161 patients (46%) died. Median survival was 31 months. We evaluated the prognostic relevance of the difference between involved (amyloidogenic) and uninvolved FLC concentration (dFLC). Median values of dFLC measured with the two methods were 180 mg/L by BS and 165 mg/L by S. Patients with dFLC greater than the median value had a worse outcome (2 year survival 43% vs. 65%, P=0.001, by BS; 42% vs. 66%, P=0.001, by S). These thresholds were incorporated into a staging system modeled on the revised Mayo Clinic system (Figure 1). The S assay provided better discrimination between stages III and IV. We evaluated the use of the S assay in the response criteria of the International Society of Amyloidosis (ISA). A serum sample collected 3 months after treatment initiation was available in 226 patients. Among them, baseline dFLC was ≥50 mg/L (evaluable for response) in 171 subjects with the BS, in 170 with the S assay, and in 146 patients by both methods. Table 2 reports the response categorization according to the two methods. The greatest discrepancy was observed in the partial response group, although also the very good partial response group presented notable deviations.
The novel monoclonal FLC assay has a diagnostic sensitivity comparable to that of the standard polyclonal assay and can be used for prognostic stratification. However, response assessment presents significant discrepancies between the two methods, indicating that further studies are needed to assess the performance of the S assay in the evaluation of response.
Diagnostic sensitivity of IFE and FLC k/l ratio by Binding Site (BS) and Siemens (S) in 338 patients with systemic AL amyloidosis
| . | Patients with k clones (N=67) . | Patients with l clones (N=271) . | Overall population (N=338) . | |||
|---|---|---|---|---|---|---|
| N positive . | % (95% CI) . | N positive . | % (95% CI) . | N positive . | % (95% CI) . | |
| Serum IFE | 55 | 82 (71–90) | 259 | 96 (93–98) | 314 | 93 (90–95) |
| Urine IFE | 54 | 81 (69–89) | 239 | 88 (84–92) | 293 | 87 (83–90) |
| Serum and urine IFE | 56 | 84 (72–91) | 262 | 97 (94–98) | 318 | 94 (91–96) |
| FLC k/l ratio BS | 65 | 97 (90–100) | 214 | 80 (74–83) | 279 | 82 (78–86) |
| FLC k/l ratio S | 60 | 89 (80–96) | 225 | 83 (78–87) | 285 | 84 (80–88) |
| Serum and urine IFE + FLC k/l ratio BS | 67 | 100 (96–100) | 264 | 97 (95–99) | 331 | 98 (96–99) |
| Serum and Urine IFE + FLC k/l ratio S | 64 | 95 (87–99) | 268 | 99 (97–100) | 332 | 98 (96–99) |
| . | Patients with k clones (N=67) . | Patients with l clones (N=271) . | Overall population (N=338) . | |||
|---|---|---|---|---|---|---|
| N positive . | % (95% CI) . | N positive . | % (95% CI) . | N positive . | % (95% CI) . | |
| Serum IFE | 55 | 82 (71–90) | 259 | 96 (93–98) | 314 | 93 (90–95) |
| Urine IFE | 54 | 81 (69–89) | 239 | 88 (84–92) | 293 | 87 (83–90) |
| Serum and urine IFE | 56 | 84 (72–91) | 262 | 97 (94–98) | 318 | 94 (91–96) |
| FLC k/l ratio BS | 65 | 97 (90–100) | 214 | 80 (74–83) | 279 | 82 (78–86) |
| FLC k/l ratio S | 60 | 89 (80–96) | 225 | 83 (78–87) | 285 | 84 (80–88) |
| Serum and urine IFE + FLC k/l ratio BS | 67 | 100 (96–100) | 264 | 97 (95–99) | 331 | 98 (96–99) |
| Serum and Urine IFE + FLC k/l ratio S | 64 | 95 (87–99) | 268 | 99 (97–100) | 332 | 98 (96–99) |
Concordance between dFLC response with the two assays
| . | Number responding by the Siemens assay . | |||
|---|---|---|---|---|
| Response by the Binding Site assay . | CR . | VGPR . | PR . | NR . |
| CR (22 patients) | 21 | 1 | 0 | 0 |
| VGPR (29 patients) | 1 | 16 | 5 | 7 |
| PR (37 patients) | 0 | 3 | 18 | 16 |
| NR (58 patients) | 0 | 3 | 3 | 52 |
| . | Number responding by the Siemens assay . | |||
|---|---|---|---|---|
| Response by the Binding Site assay . | CR . | VGPR . | PR . | NR . |
| CR (22 patients) | 21 | 1 | 0 | 0 |
| VGPR (29 patients) | 1 | 16 | 5 | 7 |
| PR (37 patients) | 0 | 3 | 18 | 16 |
| NR (58 patients) | 0 | 3 | 3 | 52 |
Complete response (CR), negative serum and urine immunofixation and normal FLC k/l ratio; very good partial response (VGPR), dFLC concentration after chemotherapy <40 mg/L; partial response (PR), dFLC decrease >50%; no response (NR), all other patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal