Abstract
Abstract 3849
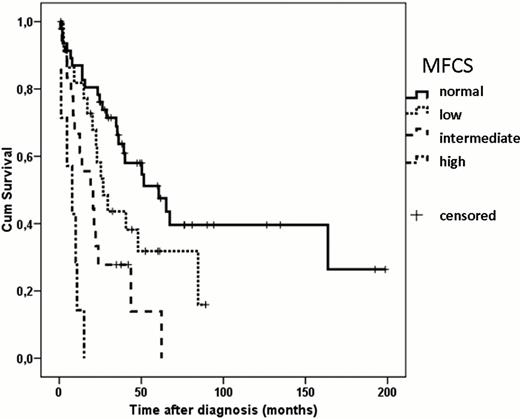
The International Prognostic Scoring System (IPSS) has found widespread application in the clinical practice of myelodysplastic syndromes (MDS) for estimation of prognosis and treatment decision making. Increasing knowledge on components that encompass the IPSS has led to the design of the revised IPSS (IPSS-R, Greenberg, Blood 2012). Furthermore, it was recognized that techniques such as flow cytometry and molecular analyses should be validated for their prognostic value in MDS; once proven the added value, they might be part of future prognostic scoring systems. Over the past years, flow cytometry based scoring systems have been proposed to refine prognostication of MDS (Wells, Blood 2003; Matarraz, Cytometry 2010). The aim of our study was to construct a simple and widely applicable hazard-adapted flow cytometry based scoring system for the prognosis of myelodysplastic syndromes and investigate whether it is of added value to the IPSS-R. Bone marrow of 96 patients with MDS was analyzed by flow cytometry. Patients were assigned to an IPSS-R category based on bone marrow blast count, cytogenetics and depth of cytopenias. Patients that received a hematopoietic stem cell transplantation or chemotherapy were censored from start of treatment. All samples were processed according to European LeukemiaNet guidelines (van de Loosdrecht, Haematologica 2009 and Leukemia 2012). Aberrancies with regard to count, expression level of markers and lineage infidelity marker expression on myeloid progenitors, B cell progenitors, maturing erythroid, monocytic and myeloid cells were analyzed. The optimal cut-off level per variable was determined based on significance and hazard ratio (HR) of overall survival (OS) and univariate analysis. In the second step, multivariate analysis was performed with the variables that had a p-value ≤0.1. The parameters that were most predictive of OS from multivariate analysis were increased CD45 expression of myeloid progenitors, asynchronous expression of CD11b on myeloid progenitors, percentage of CD34negCD117pos mature myeloid cells, percentage of mature myeloid cells with CD14 expression and CD45 expression on monocytes. In the next step, a flow cytometric scoring system based on these five parameters was constructed. Each variable was scored for in a weighed manner and the weight was determined by the size of the HR. From this a MDS flow cytometric scoring system (MFCS) was designed that identified four risk groups. i.e. patients with a normal flow score (0–0.5 points), low (1 point), intermediate (>1–2 points) and patients with a high score (>2–4.5 points). The MFCS identified patients with significantly different OS, normal score, median OS 60.6 months (range 0.6–198.6 months), vs. low score, median OS 26.8 months (range 2.5–89.2 months), vs. intermediate score, median OS 19.1 months (range 1.6–62.3 months), vs. high score, median OS 7.9 months (range 0.5–15.1 months), p<0.001. In concordance with the IPSS-R cohort, the largest number of patients was within the low risk group, 41.7% (40/96). Interestingly, within this IPSS-R risk group, the MFCS was able to identify prognostic subgroups. The median OS of IPSS-R low risk patients with a normal MFCS score was not reached, compared with low risk patients with a low MFCS, median OS 40.6 months (range 22.5–89.2 months) and compared with intermediate MFCS, median OS 19.1 months (range 12.5–43.6 months), p<0.001. In a multivariate analysis the MFCS combined with the IPSS-R were best predictive for OS rather than the scores alone (p=0.002 and p=<0.001, respectively). In conclusion, we designed a simple, five parameter MDS flow cytometric (MFCS) score based on hazard. The MFSC score was able to identify prognostic subgroups within a well defined IPSS-R risk group. Furthermore, the MFCS combined with the IPSS-R offered refined prognostication for MDS. This implies that flow cytometric analysis has an added value for prognostication and should be part of a prognostic scoring system for MDS. In a currently ongoing prospective multi center study, these new scoring models will be validated.
Alhan:Celgene: Honoraria. Ossenkoppele:Celgene: Consultancy, Honoraria. van de Loosdrecht:Celgene: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal