Abstract
Abstract  3826
3826
The International Prognostic Scoring System (IPSS) is the most widely used clinical tool for risk stratification and tailoring treatment in myelodysplastic syndromes (MDS). Outcome of patients stratified as lower risk MDS by IPSS is variable, with a subset of patients experiencing inferior than expected outcomes. Identifying patients with higher risk disease behavior is indispensible for proper implantation of disease altering therapy. The Lower Risk MD Anderson Risk Model (LR-MDAS) is a recently proposed model provides prognostic refinement to identify such patients (Garcia-Manero et al, Leukemia 2008). To validate this model, we tested the new risk model in large external single institution cohort of patients.
Data were collected retrospectively from the Moffitt Cancer Center (MCC) MDS database and chart review. The primary objective was to validate the new risk model when applied at time of initial presentation to MCC. The LR-MDAS was calculated as published using the sum of points generated from unfavorable (non-del(5q), non–diploid) cytogenetics, hemoglobin (hgb) <10g/dl, platelet count (plt) <50 k/uL or 50–200k/uL, bone marrow blast % >= 4, and age>= 60 years. Patients were divided into 3 prognostic categories. All analyses were conducted using SPSS version 15.0. (SPSS Inc, Chicago, IL). The Kaplan–Meier method was used to estimate median overall survival. Log rank test was used to compare Kaplan–Meier survival estimates between the groups. Cox regression was used for multivariable analysis.
Between January 2001 and December 2009, 479 patients with low or int-1 risk IPSS were captured by MCC MDS database. The median age was 69 years, MDS subtypes were coded as Refractory anemia (RA) 113 (24%), refractory anemia with ring sideroblasts (RARS) 73 (15%), MDS with del(5q) 19 (4%), refractory cytopenia with multi-lineage dysplasia (RCMD) 109 (23%), refractory anemia with excess blasts (RAEB) 147 (31%), and MDS-unclassified (U) 18 (4%). IPSS risk groups were low risk in 145 (30%), and intermediate-1 (int-1) 334 (70%). Only 31 patients (7%) had a poor risk karyotype by IPSS. Red blood cell transfusion dependence was documented in 42% (n=202), 22% had elevated serum ferritin ≥ 1000 ng/ml, and 45% (n=217) received azanucleoside treatment.
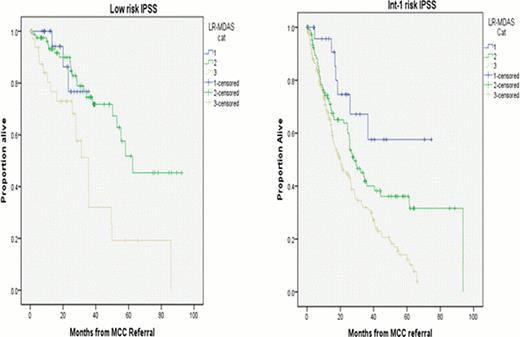
Based on the LR-MDAS, 52 patients (11%) were category 1, 188 (39%) category 2, 232 (48%) category 3, and 7 (2%) were unknown. The median OS from time of referral to MCC for all patients was 32 months (95% CI 27–37 mo), Age, IPSS risk group, serum ferritin, and RBC transfusion dependence were all significant prognostic factors in univariate analysis. The median OS for the corresponding categories was, 1 - not reached (NR), 2– 50 mo (95%CI 33–68 mo), and 3 – 22 mo (95%CI 16–27 mo), from time of MCC referral, respectively. (Figure-1) (P < 0.005).
Among 142 patients classified as low risk by IPSS, 25 patients (18%) were category 1 LR-MDAS, 81 (57%) category 2, and 36 (25%) category 3 with corresponding median OS of, NR, 62 month, and 35 month respectively (p=0.002). Among 330 patients risk stratified as int-1 IPSS group, 27 patients where category 1 LR-MDAS, 107 category 2, and 196 category 3 where median OS was NR, 28 months and 20 months, respectively. (p< 0.001) (Figure-2).
When we applied IPSS risk stratification among each category of LR-MDAS to assess if IPSS can further refine prognosis within LR-MDAS categories, only in patients classified as Category 2 LR-MDAS the median OS was different among low and int-1 risk IPSS (62 month versus 28 month). (p <0.005).
The rate of AML transformation according to LR-MDAS was 4%, 12%, and 20% for category 1,2, and 3, respectively. (p<0.02). In Cox regression analysis higher risk LR-MDAS predicted inferior OS (Hazard ratio (HR) 1.8 (95%CI 1.4–2.3) (p <0.005) independent of IPSS risk group (HR 2 95%CI 1.4–2.8) (p <0.005).
Our data validates the prognostic value of the proposed LR-MDAS risk model, demonstrating predictive power for overall survival and AML transformation among low/int-1 risk IPSS. The LR-MDAS is complementary to the IPSS, offering further discrimination to identifying those patients with aggressive disease behavior that merit disease altering therapy. The utility of the model as treatment decision tool should be studied prospectively.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal