Abstract
Abstract 3758
The EUTOS score has been proposed by the European LeukemiaNet (ELN) as a new scoring system which is predictive for 18-month (mth) complete cytogenetic response (CCyR) and 5-year (yr) progression-free survival (PFS) in chronic phase (CP) chronic myeloid leukemia (CML) patients treated with first-line imatinib (IM) (Hasford et al, Blood 2011). The score is calculated using spleen size and basophil percentage and divides patients into low risk and high risk groups. However the EUTOS score was not validated in two studies, one which determined 8-yr overall survival (OS), PFS, CCyR and major molecular remission (MMR) (Marin et al, J Clin Oncol 2011); and in another which analysed 3-yr event-free survival, transformation-free survival and OS and overall CCyR and MMR (Kantarjian H et al, Blood 2012). Recent reports have suggested that Asian CML patients may have clinical and genetic differences compared to Causcasians, e.g. younger median age at presentation (Au et al, Int J Hem 2009) and genetic polymorphism leading to IM resistance (Pan et al, Nat Med 2012). Although a different disease, splenomegaly was also reported to be less frequent in Chinese patients with myelofibrosis (Xiao et al, Blood 2012). Given these differences, we sought to determine if the EUTOS score was predictive for clinical outcome and survival in Asian CP-CML patients treated with IM.
A retrospective analysis was undertaken of CP-CML patients followed up in our institution from 2000–2012. All patients were treated with IM 400 mg within one year of diagnosis. The rates of 6-mth major cytogenetic response (MCyR), 12-mth CCyR, 18-mth CCyR, 12-mth MMR and 18-mth MMR were evaluated. MMR was defined as BCR-ABL transcript levels ≤ 0.1% by the International Scale. The probability of OS, PFS and failure-free survival (FFS) at 5 and 8 years was also determined. Progression was defined as transformation to accelerated or blast phase (AP/BP) or death from any reason. Failure was defined according to the 2009 ELN criteria or as an increase in dose of IM, change of therapy, transformation to AP/BP or death.
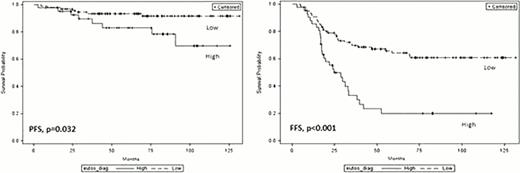
A total of 139 patients were included in the analysis. The median age at presentation of CML was 45 yrs (range 16–88) with 64% Chinese, 17% Malays and 8% Indians. There was 69% in the low risk EUTOS group. Cytogenetic responses were significantly better in the low risk group compared to the high risk group with 6-mth MCyR rates of 82% vs 48% (p<0.001), 12-mth CCyR rates of 68% vs 39% (p=0.008) and 18-mth CCyR rates of 73% vs 36% (p=0.003). MMR rates were also higher in the low risk group at 12 mth (42% vs 14%, p=0.026) and at 18 mth (56% vs 21%, p=0.009). The probability of PFS was significantly higher in the low risk group compared to the high risk group at 5 yrs (93% vs 83%, p=0.032) and at 8 yrs (92% vs 70%, p=0.032). The low risk group also had a significantly higher FFS at 5 yrs (64% vs 20%, p<0.001) and at 8 yrs (61% vs 20%, p<0.001). (Figure 1) There was a trend towards a better overall survival in the low risk group at 5 and 8 yrs but this did not reach statistical significance (95% vs 92% and 94% vs 83% respectively, p=0.084).
Our analysis confirms that the EUTOS score is a valid tool in predicting 18-mth CCyR and 5-yr PFS in Asian patients with early CP-CML treated with IM. In addition, we have shown that the EUTOS score was highly predictive for cytogenetic and molecular responses at earlier time points and long-term PFS and FFS.
Chuah:Novartis, Bristol Myers-Squibb: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal