Abstract
Abstract 3740
Based upon dramatic improvement in major and complete cytogenetic response with imatinib (STI571) in the phase III International Randomized Study of Interferon Versus STI571 (IRIS) trial, imatinib was approved for first-line treatment in CML. Despite this success, five-year follow up of this trial showed only 69% of patients remained on imatinib. Other long-term studies suggest that up to 50% of patients may require interruption or discontinuation of imatinib due to primary or secondary resistance, drug intolerance, or progression to accelerated phase (AP) or blast crisis (BC).
The Total Cancer Care (TCC) database was used to identify all patients with CML treated with imatinib as first-line therapy between 1992 and 2010 at Moffitt Cancer Center (MCC). “Imatinib-refractory” was defined as absence of complete hematologic response by 3 months, or cytogenetic response by 6 months. “Loss of response” was defined as loss of best response while remaining on the dose of imatinib that was previously therapeutic. Progression-free survival (PFS) was defined as time from treatment to loss of best response, or progression to AP or BC. Descriptive data were reported, chi square test was used for categorical variables, and Kaplan Meier method was used for OS and PFS. Log rank test was used to compare survival times between groups. Statistical analysis was done using SPSS statistical software, version 19.
Of a total of 540 CML patients evaluated, 304 (56%) received imatinib as first-line therapy, with 51% being male and 49% female. Five patients were diagnosed before 2001 and median age was 49.5 years. Median time on imatinib was 23 months. With a median follow up of 72 months (95% CI 67–76), the 5-year OS was 78% and median OS was not reached. Of 304 patients, 139 (46%) required a change to second generation TKI's. Causes for imatinib discontinuation included: intolerance in 48 (35%) patients, refractory disease in 34 (24%) patients, loss of response in 26 (19%) patients, and progression to AP or BC in 31 (22%) patients.
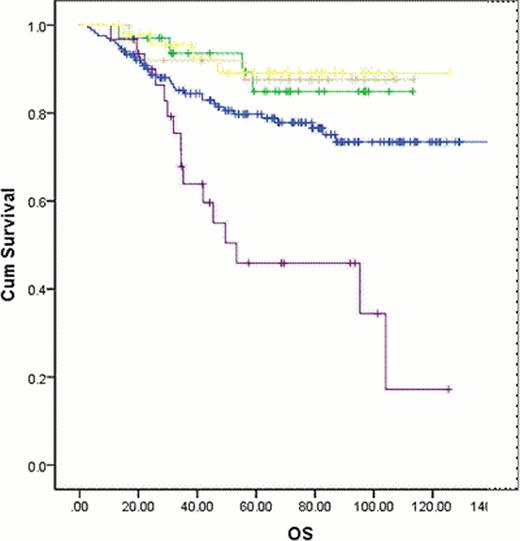
In patients switched due to intolerance, refractory disease or loss of response median PFS and OS were not reached. Median OS was 53 months (95% CI 5.4–101.3) in patients whose therapy was changed due to progression to AP or BC (P=0.000) (Figure 1). Overall, 82 (27%) patients progressed to AP or BC either on first or second line therapy, and 25 (8%) developed kinase domain mutations (KDM). Of the 25 patients with KDM, 13 (52%) developed T315I mutations.
Despite the success of imatinib in improving outcomes in CML, nearly half of all patients will require discontinuation of the drug with a change to second line TKI's. This change does not appear to negatively impact OS, with the exception of therapy changes due to overt progression into AP or BC. Our results suggest that most imatinib failures may be salvaged with second generation TKI's without significant impact upon overall survival.
OS in all patients based on reason for discontinuing imatinib
OS in all patients based on reason for discontinuing imatinib
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal