Abstract
Abstract 3696
The majority of patients (pts) with mantle cell lymphoma (MCL) respond to initial treatment, yet most pts relapse and die from disease. Although stem cell transplantation (SCT) is a treatment option, it is not always curative and many pts are not eligible. Based upon single agent activity of thalidomide and bortezomib in MCL (overall response rates [ORR] approximately 30%), CALGB designed a phase II trial of bortezomib + lenalidomide therapy for pts with relapsed/refractory MCL.
Eligibility criteria included: histologically confirmed MCL (CD5+, CD 23-, cyclin D1+); measurable disease; prior therapy with >1 regimen that may include autologous (not allogeneic) SCT, now with relapsed/refractory disease; no prior radioimmunotherapy, bortezomib, or lenalidomide; ECOG performance status (PS) 0–2; no >grade (gr) 3 peripheral neuropathy. Induction therapy was lenalidomide (20 mg po qd, days [D] 1–14) plus bortezomib (1.3 mg/m2 IV, D 1, 4, 8, 11), given every 21 D for 8 cycles. Pts were restaged at 3 & 6 mos; responding patients (complete and partial responses; [CR+PR]) at 6 mos received maintenance with lenalidomide (15 mg po D 1–14) and bortezomib (1.3 mg/m2 IV, D 1, 8), until disease progression (PD). In 9/09, the protocol was modified to have separate toxicity-related dose reductions for each agent (myelosuppression, decrease lenalidomide; neuropathy, decrease bortezomib). Primary endpoints were ORR (=CR+PR), CR rate; secondary endpoints were event-free- (EFS) (time to progression, all-cause death, or initiation of non-protocol therapy), progression-free- (PFS) (time to progression or all-cause death), and overall survival (OS) (time to death from any cause). The study design considered an ORR <45% too low, and >65% of strong interest. Response and toxicity data were summarized using frequency tables; Kaplan-Meier method was used to analyze survival parameters. The study was activated in 11/07. It temporarily closed for planned interim analysis after accrual of 19 pts, and closed in 7/11, when it reached the accrual goal of 54 pts.
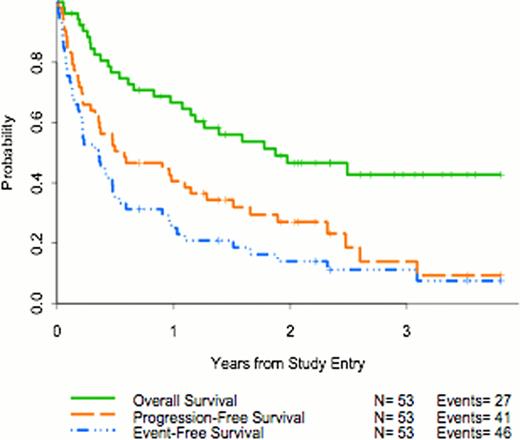
Median pt age was 67 (range, 39–83) yrs; 83% were male. 47 (89%) pts had relapsed, and 6 (11%) refractory, disease. 79% of pts had stage IV disease; 21% had B-symptoms; lactate dehydrogenase was elevated in 17 (32%). PS was 0 (62%), 1 (36%), and 2 (2%). Number of prior chemotherapy regimens was: one (60% of pts), 2 (25%), 3 (12%), 4 (2%), and 5 (2%). Prior therapy included rituximab in 46 (85%), radiotherapy in 14 (26%), and SCT in 21 (40%). Median number of protocol therapy cycles received was 4 (range, 1–64); 20 pts (37%) received <2 cycles. Reasons for treatment discontinuation included: PD in 20 pts (38%); no response in 1 (2%); toxicity in 17 (32%); pt refusal in 4 (8%); rising lymphocyte count in 1; physician decision in 1; other treatment in 6 (8%). Best response for the 53 eligible pts was 8 (15%) CR, 13 (25%) PR, 8 (15%) stable disease (SD), 17 (32%) PD; 7 (13%) unevaluable pts were taken off treatment after cycle one. Among the responding pts, 5/8 CR pts and 4/13 PR pts went onto maintenance therapy. Of the responders, 6 CR and 1 PR pts remain in remission. 27 (51%) pts have died (1 treatment-related, 24 with PD, 2 with infections). Median follow-up in the 26 living eligible pts is 2.3 (range, 0.1–3.8) yrs. One-yr EFS, PFS, and OS are 25%, 41%, and 67%, respectively.
Gr 3/4 toxicities (% gr 3/% gr 4) were: anemia (8/0), leukopenia (6/0), thrombocytopenia (13/21), fatigue/aesthenia (21/0), dyspnea (9/0), infection (6/0), motor neuropathy (9/0), sensory neuropathy (8/0; gr 2 36%), hypotension (8/0), and thrombosis (6/0). During induction therapy (cycles 1–8), at least >1 lenalidomide or bortezomib dose reduction occurred in 24 (44%) and 29 (54%) pts, respectively, due to adverse events; 21 (39%) pts had dose reductions in both drugs.
The ORR of 40% for the combination of lenalidomide + bortezomib was disappointing given the high single agent response rates reported with lenalidomide. The lower than anticipated ORR may be due to inadequate lenalidomide dosing, initial simultaneous dose reductions in both agents for toxicity, or the limited protocol therapy administered in 37% of pts. Due to the small number of pts receiving maintenance therapy, one cannot assess its impact. Future studies with this dose and schedule of lenalidomide + bortezomib are not warranted.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal