Abstract
Abstract 3677
MEDI-551 is an affinity-optimized and afucosylated humanized IgG kappa monoclonal antibody directed against CD19 and induces malignant clone destruction by antibody-dependent cellular cytotoxicity. This study evaluates the safety and preliminary efficacy profile of MEDI-551 in patients with relapsed/refractory B-cell malignancies. These include chronic lymphocytic leukemia (CLL), diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), and multiple myeloma (MM).
To determine the safety profile and maximum tolerated dose (MTD) or optimal biological dose of MEDI-551 in patients with relapsed/refractory B-cell malignancies. Secondary objectives include pharmacokinetics, immunogenicity, and clinical activity of MEDI-551.
In this phase 1/2 open-label multicenter, global dose-escalation and expansion study, patients with relapsed or refractory CLL, DLBCL, FL, or MM received MEDI-551 (at 0.5, 1, 2, 4, 8, or 12 mg/kg) by intravenous infusion administered over 28-day cycles using standard 3+3 dose escalation. Dose escalation continued until the MTD (≤12 mg/kg) was reached. Therapy continued for 2 cycles beyond complete response (CR), or until unacceptable toxicity or disease progression. Dose-limiting toxicity was defined as a MEDI-551-related adverse event (AE) that prevented completion of a full first cycle of MEDI-551, or as a grade 3 or higher toxicity (excluding hematologic toxicity) that could not be ascribed to another cause, such as disease progression or accident.
Of the 63 patients (CLL [12], DLBCL [23], FL [23], MM [5]) who received ≥1 dose of MEDI-551, 25 patients (CLL [3], DLBCL [6], FL [12], MM [4]) were enrolled in the phase 1 escalation portion (Jun 2010–Aug 2011). No MTD was achieved. The phase 2 expansion phase is ongoing. Median age of patients treated was 62 years. The median of completed treatment cycles was 4.0 with a maximum of 15 cycles. Dose intensity was approximately 98%. There were 6 deaths due to AEs (none were drug-related) and 9 subjects discontinued treatment (2 [neutropenia and infusion site reaction] were nonserious drug-related AEs). Most AEs were grade 1/2 with dose-independent frequency and severity (Table). 13 patients had serious treatment-emergent adverse events (TEAEs); pneumonia, sepsis, and bacteremia in 1 patient were considered drug-related. 7 patients had grade 4 TEAEs (2 with neutropenia were drug-related) and 7 had grade 5 events, none related to drug.
Treatment-emergent adverse events with highest severity by frequency (safety population)
| Adverse Event . | Patients, n (%) (N=63) . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . |
|---|---|---|---|---|---|
| Fatigue | 16 (25.4) | 9 (14.3) | 7 (11.1) | – | – |
| Diarrhea | 14 (22.2) | 12 (19.0) | 1 (1.6) | 1 (1.6) | – |
| Infusion reaction | 12 (19.0) | 2 (3.2) | 10 (15.9) | – | – |
| Nausea | 11 (17.5) | 9 (14.3) | 2 (3.2) | – | – |
| Cough | 9 (14.3) | 8 (12.7) | 1 (1.6) | – | – |
| Neutropenia | 9 (14.3) | – | 3 (4.8) | 3 (4.8) | 3 (4.8) |
| Thrombocytopenia | 9 (14.3) | 1 (1.6) | 5 (7.9) | 3 (4.8) | – |
| Dyspnea | 8 (12.7) | 7 (11.1) | 1 (1.6) | – | – |
| Headache | 8 (12.7) | 6 (9.5) | 2 (3.2) | – | – |
| Vomiting | 8 (12.7) | 6 (9.5) | 2 (3.2) | – | – |
| Abdominal pain | 7 (11.1) | 6 (9.5) | – | 1 (1.6) | – |
| Pyrexia | 7 (11.1) | 6 (9.5) | 1 (1.6) | – | – |
| Anemia | 6 (9.5) | 1 (1.6) | 4 (6.3) | 1 (1.6) | – |
| Decreased appetite | 6 (9.5) | 6 (9.5) | – | – | – |
| Adverse Event . | Patients, n (%) (N=63) . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . |
|---|---|---|---|---|---|
| Fatigue | 16 (25.4) | 9 (14.3) | 7 (11.1) | – | – |
| Diarrhea | 14 (22.2) | 12 (19.0) | 1 (1.6) | 1 (1.6) | – |
| Infusion reaction | 12 (19.0) | 2 (3.2) | 10 (15.9) | – | – |
| Nausea | 11 (17.5) | 9 (14.3) | 2 (3.2) | – | – |
| Cough | 9 (14.3) | 8 (12.7) | 1 (1.6) | – | – |
| Neutropenia | 9 (14.3) | – | 3 (4.8) | 3 (4.8) | 3 (4.8) |
| Thrombocytopenia | 9 (14.3) | 1 (1.6) | 5 (7.9) | 3 (4.8) | – |
| Dyspnea | 8 (12.7) | 7 (11.1) | 1 (1.6) | – | – |
| Headache | 8 (12.7) | 6 (9.5) | 2 (3.2) | – | – |
| Vomiting | 8 (12.7) | 6 (9.5) | 2 (3.2) | – | – |
| Abdominal pain | 7 (11.1) | 6 (9.5) | – | 1 (1.6) | – |
| Pyrexia | 7 (11.1) | 6 (9.5) | 1 (1.6) | – | – |
| Anemia | 6 (9.5) | 1 (1.6) | 4 (6.3) | 1 (1.6) | – |
| Decreased appetite | 6 (9.5) | 6 (9.5) | – | – | – |
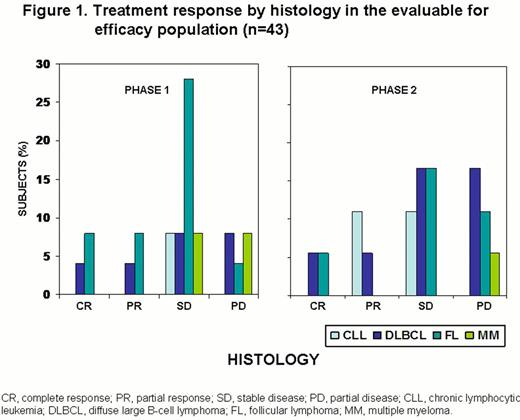
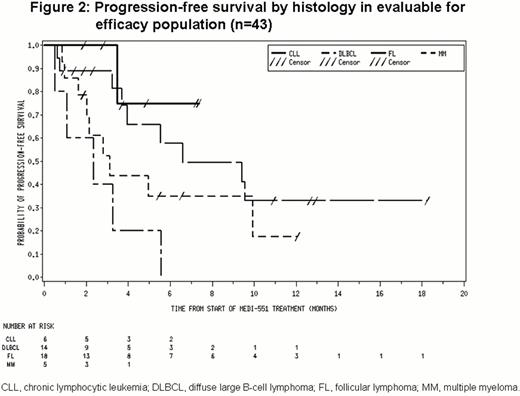
Of 43 patients in the evaluable for efficacy population (includes all patients who received any treatment of MEDI-551 and completed at least 1 post-baseline disease assessment), 5 had CR, 6 had partial responses (PR) and 21 had stable disease (SD; Figure 1). Median progression-free survival was ≈6 months (Figure 2).
MEDI-551 demonstrated a safety profile warranting further study and no MTD was identified at the highest dose studied. The responses (CR: 11.6%; PR: 14.0%, objective response of 25.6% and SD of 48.8%) achieved in this single-agent study in heavily pretreated patients provide encouraging evidence of antitumor activity.
Fanale:MedImmune: Research Funding. Kipps:MedImmune: Membership on an entity's Board of Directors or advisory committees, Research Funding. Gregory:MedImmune (ALLRESEARCH FINDING PROCEEDS GO TO RUSH UVERSITY MEDICAL CENTER, NOT TOT ME PERSONALLY: Honoraria, Research Funding. Zhang:MedImmune: Employment. Goswami:MedImmune: Employment; AstraZeneca: Stocks, Stocks Other. Ibrahim:MedImmune: Employment; AstraZeneca: Stocks, Stocks Other. Yao:MedImmune: Employment. Herbst:MedImmune: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal