Abstract
Abstract 3659
Combined modality therapy with chemotherapy and radiation (RT) has resulted in excellent cure rates for Hodgkin Lymphoma (HL) but is associated with significant late effects. Modern therapeutic protocols attempt to limit cumulative doses of chemotherapy and minimize radiation therapy where possible. There is also growing attention to the optimal management of specific patient populations, including the adolescent and young adult (AYA) population. The CCG trial 5942 provides an opportunity to assess the role of RT in disease control for the AYA population (15–21 years of age) compared with a younger cohort (<15 years of age).
The CCG 5942 study included 826 eligible patients (339 AYA) accrued during the years 1995–1998. Patients were assigned to risk-adapted chemotherapy based on stage and presence or absence of unfavourable risk factors (B symptoms, bulk disease, hilar lymphadenopathy, number of involved nodal sites). Disease response was assessed at the completion of chemotherapy and patients in CR were eligible for randomization to receive 21 Gy IFRT or no further therapy. We conducted a retrospective analysis of the CCG 5942 data to compare demographics, treatment response and survival outcomes of children and AYA.
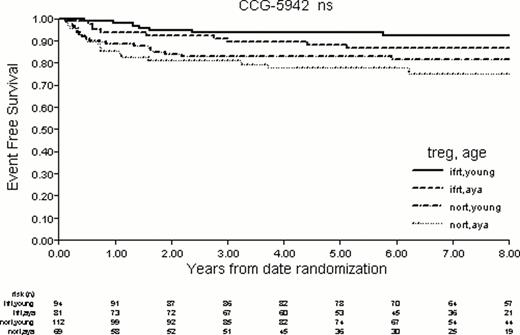
Patient demographics are shown in Table 1. The AYA patients were more likely to present with nodular sclerosing histology (NS), B symptoms, mediastinal bulk disease, and higher stage. The CR rates for children and AYA were 393/487 (80.7%) and 243/339 (71.7%) (p=.003). Of the 636 patients in CR, 498 (181 AYA) participated in the randomization. The AYA patients were less likely to be compliant with randomization to no further therapy (2/165 children vs 5/84 AYA, p=.045). The 8y EFS and OS for all children compared to AYA were 85.7% vs 80.2% (p=.052) and 94.3% vs 90.7% (p=.060) respectively. The 8y EFS and OS for all randomized children compared to AYA were 89.8 vs 81.3 (p=.011) and 97.2% vs 95.1% (p=0.30). A Cox model adjusting for both age group and histology (NS vs. lymphocyte predominant (LP) and mixed cellularity (MC)) demonstrated age to be an insignificant predictor (p=0.30) and NS histology to be a significant predictor (p=.001, HR 2.6) of worse EFS. EFS for all randomized patients with NS histology by age group and treatment received are shown in Figure 1. There is a significant difference among the groups in the overall comparison (p=0.018). There was no difference in OS with all pairwise comparisons p>0.5.
The AYA were more likely to present with higher risk disease and less likely to enter CR compared to younger children on the CCG 5942 trial. Among randomized patients with NS histology, both young and AYA populations show an EFS advantage with RT, though this advantage does not reach statistical significance for the AYA group. There was no difference in OS for randomized patients with NS histology in either age group. This analysis is limited by the retrospective methodology but supports further study of therapy assignment of the AYA population. Elimination of RT from AYA with NS histology may prove more challenging than for younger children. Analysis of current protocols using a uniform chemotherapy backbone and early disease response assessment with PET/CT will be particularly informative.
Patient characteristics
| . | All patients (n=826) . | Randomized patients (n=498) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <15 yrs . | 15–21 yrs . | p-value . | <15 yrs . | 15–21yrs . | p-value . | |||||
| N . | % . | n . | % . | n . | % . | n . | % . | |||
| Gender | <0.001 | 0.005 | ||||||||
| male | 294 | 60% | 160 | 47% | 192 | 61% | 86 | 48% | ||
| female | 193 | 40% | 179 | 53% | 125 | 39% | 95 | 52% | ||
| Histology | <0.001 | <0.001 | ||||||||
| LP | 65 | 14% | 18 | 5% | 45 | 14% | 10 | 6% | ||
| NS | 322 | 68% | 290 | 88% | 206 | 66% | 150 | 84% | ||
| MC | 85 | 18% | 20 | 6% | 58 | 19% | 16 | 9% | ||
| LD | 3 | 0.6% | 2 | 0.6% | 2 | 0.6% | 2 | 1% | ||
| Unknown | 12 | 9 | 6 | 3 | ||||||
| Stage | <0.001 | 0.001 | ||||||||
| I | 104 | 21% | 31 | 9% | 79 | 25% | 20 | 11% | ||
| II | 231 | 47% | 200 | 59% | 150 | 47% | 111 | 61% | ||
| III | 71 | 15% | 49 | 14% | 46 | 15% | 26 | 14% | ||
| IV | 81 | 17% | 59 | 17% | 42 | 13% | 24 | 13% | ||
| B-sym | 0.016 | 0.002 | ||||||||
| present | 109 | 22% | 101 | 30% | 54 | 17% | 52 | 29% | ||
| absent | 378 | 78% | 238 | 70% | 263 | 83% | 129 | 71% | ||
| Mediastinal Mass | <0.001 | 0.002 | ||||||||
| >=1/3 | 104 | 21% | 101 | 30% | 58 | 18% | 40 | 22% | ||
| <1/3 | 181 | 37% | 152 | 45% | 117 | 37% | 89 | 49% | ||
| no | 202 | 41% | 86 | 25% | 142 | 45% | 52 | 29% | ||
| Clinical Group | 0.003 | 0.017 | ||||||||
| 1 | 191 | 39% | 95 | 28% | 146 | 46% | 61 | 34% | ||
| 2 | 214 | 44% | 183 | 54% | 129 | 41% | 96 | 53% | ||
| 3 | 82 | 17% | 61 | 18% | 42 | 13% | 24 | 13% | ||
| . | All patients (n=826) . | Randomized patients (n=498) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <15 yrs . | 15–21 yrs . | p-value . | <15 yrs . | 15–21yrs . | p-value . | |||||
| N . | % . | n . | % . | n . | % . | n . | % . | |||
| Gender | <0.001 | 0.005 | ||||||||
| male | 294 | 60% | 160 | 47% | 192 | 61% | 86 | 48% | ||
| female | 193 | 40% | 179 | 53% | 125 | 39% | 95 | 52% | ||
| Histology | <0.001 | <0.001 | ||||||||
| LP | 65 | 14% | 18 | 5% | 45 | 14% | 10 | 6% | ||
| NS | 322 | 68% | 290 | 88% | 206 | 66% | 150 | 84% | ||
| MC | 85 | 18% | 20 | 6% | 58 | 19% | 16 | 9% | ||
| LD | 3 | 0.6% | 2 | 0.6% | 2 | 0.6% | 2 | 1% | ||
| Unknown | 12 | 9 | 6 | 3 | ||||||
| Stage | <0.001 | 0.001 | ||||||||
| I | 104 | 21% | 31 | 9% | 79 | 25% | 20 | 11% | ||
| II | 231 | 47% | 200 | 59% | 150 | 47% | 111 | 61% | ||
| III | 71 | 15% | 49 | 14% | 46 | 15% | 26 | 14% | ||
| IV | 81 | 17% | 59 | 17% | 42 | 13% | 24 | 13% | ||
| B-sym | 0.016 | 0.002 | ||||||||
| present | 109 | 22% | 101 | 30% | 54 | 17% | 52 | 29% | ||
| absent | 378 | 78% | 238 | 70% | 263 | 83% | 129 | 71% | ||
| Mediastinal Mass | <0.001 | 0.002 | ||||||||
| >=1/3 | 104 | 21% | 101 | 30% | 58 | 18% | 40 | 22% | ||
| <1/3 | 181 | 37% | 152 | 45% | 117 | 37% | 89 | 49% | ||
| no | 202 | 41% | 86 | 25% | 142 | 45% | 52 | 29% | ||
| Clinical Group | 0.003 | 0.017 | ||||||||
| 1 | 191 | 39% | 95 | 28% | 146 | 46% | 61 | 34% | ||
| 2 | 214 | 44% | 183 | 54% | 129 | 41% | 96 | 53% | ||
| 3 | 82 | 17% | 61 | 18% | 42 | 13% | 24 | 13% | ||
EFS of all Randomized NS Patients by Age Group and Treatment Received. Pair-wise comparisons: IFRT/Young vs. noRT/Young (p=0.023); IFRT/Young vs. noRT/AYA (p=0.002); IFRT/AYA vs. noRT/AYA (p=0.068); all other comparisons, p > 0.2.
EFS of all Randomized NS Patients by Age Group and Treatment Received. Pair-wise comparisons: IFRT/Young vs. noRT/Young (p=0.023); IFRT/Young vs. noRT/AYA (p=0.002); IFRT/AYA vs. noRT/AYA (p=0.068); all other comparisons, p > 0.2.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal