Abstract
Abstract 350
Relapse is the leading cause of death following allogeneic hematopoietic cell transplant (HCT) for hematological malignancies. Although evidence suggests that the beneficial donor T cell-mediated graft versus leukemia (GVL) effect can reduce post-HCT relapse rates, this is often mitigated by morbidity and mortality associated with the accompanying graft versus host disease (GVHD). Thus, providing antigen-specific T cells that selectively target leukemia associated antigen (LAA) constitutes a distinct opportunity to promote GVL activity without inducing GVHD. Wilms' Tumor Antigen 1 (WT1) is a non-polymorphic zinc finger transcription factor that plays a key role in cell growth and differentiation. WT1 has a very limited expression in normal tissues, is expressed 10–1000× fold more in leukemia cells compared to normal CD34+ cells, and has been shown to contribute to leukemogenesis. WT1 is expressed in acute myeloid leukemia (AML), myelodysplastic syndrome (MDS), chronic myeloid leukemia (CML) and acute lymphoid leukemia (ALL). Furthermore, the magnitude of expression of WT1 in leukemic cells correlates with prognosis and clinical aggressiveness. Thus, WT1 constitutes an attractive candidate target for CD8+cytotoxic T-cells (CTL) (Cheever et al. Clin Cancer Res 2009;15(17):5323–5337).
In this study, escalating doses of clonal populations of donor-derived CD8+ CTL specific for the HLA A*02:01-restricted WT1126–134 (RMFPNAPYL) epitope were administered to 11 high-risk leukemia patients after allogeneic HCT. The absence of end-organ toxicities or the development of new-onset GVHD demonstrated that the infusions were safe and well-tolerated. As the persistence of transferred cells was limited in some patients, the last four patients received CTL clones primed in the presence of the γc-chain cytokine Interleukin-21 (IL-21), a culture strategy recently shown to confer a less differentiated phenotype to T cells generated in vitro, as a means to increase the ability of transferred cells to survive in vivo and potentially mediate greater anti-leukemic activity (Li, Y et al. J Immunol 2005;175:2261–2269).
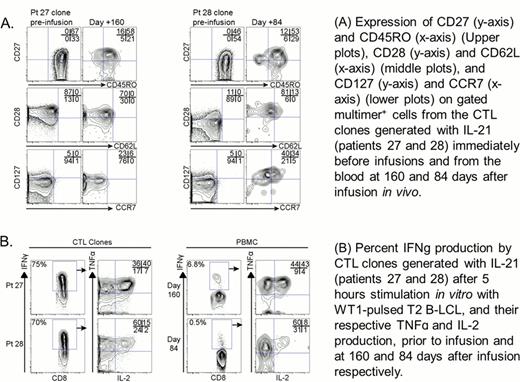
Four patients, who were treated not in florid relapse (3 in CR and 1 with MRD entering infusions) but were at high risk for relapse post-HCT (40–55% relapse rate at one year post HCT), and received CTL generated in the presence of IL-21 have survived for 22 to 37 months post-HCT without detectable leukemia or relapse, and in the absence of additional anti-leukemic treatment or GVHD (Table 1). In these four patients, transferred CTL remained detectable for 8 to 15 month after T cell infusions (Fig. 1), and maintained/upregulated in vivo phenotypic (CD27, CD28, CD127, CD62L and CCR7) and functional (the ability to produce IL-2 in response to cognate antigen) characteristics associated with long-lived memory CD8 T-cells (Fig. 2). Direct evidence of transient anti-leukemic activity was observed in one patient treated with advanced progressive disease, and of a prolonged response in a patient with minimal residual disease.
The results of this study suggest that transfer of donor-derived WT1-specific CTL clones can be accomplished without significant toxicity and can potentially provide therapeutic anti-leukemic activity.
In vivo persistence of WT1-specific CTL clones and effect on leukemia disease burden
In vivo persistence of WT1-specific CTL clones and effect on leukemia disease burden
Adoptively transferred WT1-specific CD8+T-cells persisting in vivo exhibit many phenotypic and functional characteristics associated with CD8+central memory cells
Adoptively transferred WT1-specific CD8+T-cells persisting in vivo exhibit many phenotypic and functional characteristics associated with CD8+central memory cells
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal