Abstract
Abstract 3472
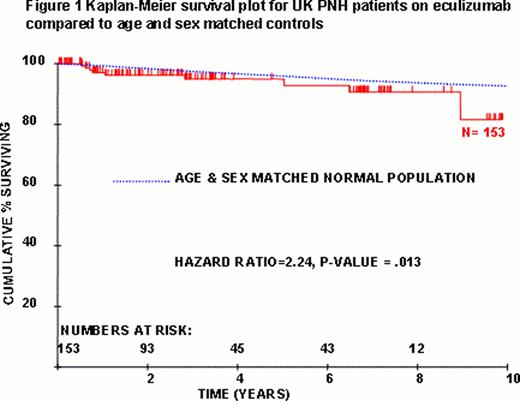
Paroxysmal Nocturnal Hemoglobinuria (PNH) is an acquired clonal stem cell disorder arising on the background of bone marrow failure and resulting in hemolytic anemia, thrombosis, pulmonary hypertension (PHT) and chronic kidney disease (CKD) through uncontrolled complement activation. Approximately half of patients treated with supportive therapies alone die as a result of their PNH. Eculizumab blocks C5 and thereby terminal complement activation. Complications are therefore prevented with reduction in intravascular hemolysis, transfusion requirements, thromboses, pulmonary pressures and renal impairment all associated with significantly improved quality of life. The UK has a nationally commissioned PNH service led by 2 Centres, Leeds and King's. Between May 2002 - April 2012, a total of 153 patients (76 male; 77 female) were treated with eculizumab for PNH in the UK for a mean of 42 mths (0.4 – 119). Patients are treated if they have 1) transfusion-dependent hemolysis or 2) independent of transfusions, either i) thrombosis, ii) CKD, iii) PHT, iv) pregnancy or v) LDH>1.5× upper limit normal with anemia and symptoms including fatigue, dysphagia, dyspnea or abdominal pain due to PNH. Median age at PNH diagnosis was 34 yrs (range 12–80) and at initiation of eculizumab was 42 yrs (range 14–84). There were 28 thrombotic episodes in 15/65 (23%) patients anticoagulated prior to commencing eculizumab therapy. Nine patients have died - 3 due to progression of their underlying bone marrow failure to MDS/AML, one died immediately after BMT and the remaining 5 deaths are not directly related to PNH. Seven patients discontinued eculizumab - one had predominant aplastic anemia (AA), 2 had spontaneous remissions of the PNH clone, 3 commenced for a pregnancy discontinuing after and one who had a successful transplant for very severe AA. One hundred and thirty-seven patients remain on eculizumab. There were 36 thrombotic episodes in 22 patients in the 12 months prior to eculizumab and 3 thromboses in 3 patients in the most recent 12 months on therapy (1 Budd-Chiari during complement blockade breakthrough caused by infection, 1 CVA during reversal of coumadin overanticoagulation, 1 TIA/lacunar infarct thought to be due to diabetic small vessel disease) (P<0.05). Of the 22 patients who had a thrombosis within 12 months prior to starting eculizumab there were no further thrombotic episodes once on eculizumab. Importantly, primary and secondary prophylaxis with warfarin has been safely stopped in 43 and 4 patients respectively, with no thrombotic sequelae. Ten patients had not required a transfusion before receiving eculizumab. Of the 117 patients transfused in the 12 months before receiving eculizumab and on therapy for the most recent 12 months, 77 (66%) became transfusion independent. Of the 40 patients still needing transfusions, there was a significant (P<0.05) reduction in the number of units transfused from a median of 26 units 12 months before therapy to 8 units in the most recent 12 months on therapy. There were 3 cases of meningococcal septicaemia out of 153 patients treated (0.6 cases/100 patient yrs on therapy) with none in the last 2 yrs since routine penicillin prophylaxis was instituted. All were promptly and effectively managed and remain on eculizumab therapy. The survival of UK PNH patients on eculizumab was compared with age and sex matched controls (Fig 1) and demonstrates improved survival compared with historical controls. Survival after 10 years is slightly inferior to controls with causes of death related to bone marrow failure and not hemolysis or thrombosis.
the total UK cohort provide more than 10 years experience of eculizumab for PNH and shows a) continuing safety and efficacy of eculizumab with persistent significant improvement in symptoms and quality of life b) no evidence of tolerance or refractoriness c) significant reduction in thrombosis risk on eculizumab therapy and safety in discontinuing primary anticoagulation d) significant reduction in the need for transfusions e) progression to MDS/AML as expected for the underlying bone marrow failure and not considered related to eculizumab and f) significant improvement in survival for PNH patients receiving eculizumab. This cohort of all PNH patients treated with eculizumab in the UK demonstrates the impact of eculizumab in the quality of life, reduction in complications and thereby improved survival for patients.
Hill:Alexion Pharmaceuticals, Inc.: Consultancy, Honoraria. Kelly:Alexion Pharmaceuticals, Inc.: Consultancy, Honoraria. Gandhi:Alexion Pharmaceuticals, Inc.: Research Funding. Mitchell:Alexion Pharmaceuticals, Inc: Honoraria. Elebute:Alexion Pharmaceuticals, Inc.: Consultancy, Honoraria, Research Funding. Arnold:Alexion Pharmaceuticals, Inc.: Honoraria. Marsh:Alexion Pharmaceuticals, Inc.: Honoraria. Hillmen:Alexion Pharmaceuticals, Inc: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal