Abstract
Abstract 3458
The interaction between hematopoietic cells and bone marrow microenvironment is important for the regulation of hematopoiesis. Recent studies have identified the specific bone marrow microenvironment for the hematopoietic stem cells, the “endosteal niche” and the “vascular niche”, in which mesenchymal stem cells and their progenies including osteoblasts are demonstrated to be major cellular constituents. We found that osteogenesis-induced mesenchymal stem cells by the combinations of ascorbic acid, dexamethasone, and potassium dihydrogen phosphate have unique capabilities of both expanding CD34+ hematopoietic progenitor cells and of differentiating CD34+ hematopoietic progenitor cells into mature cells.
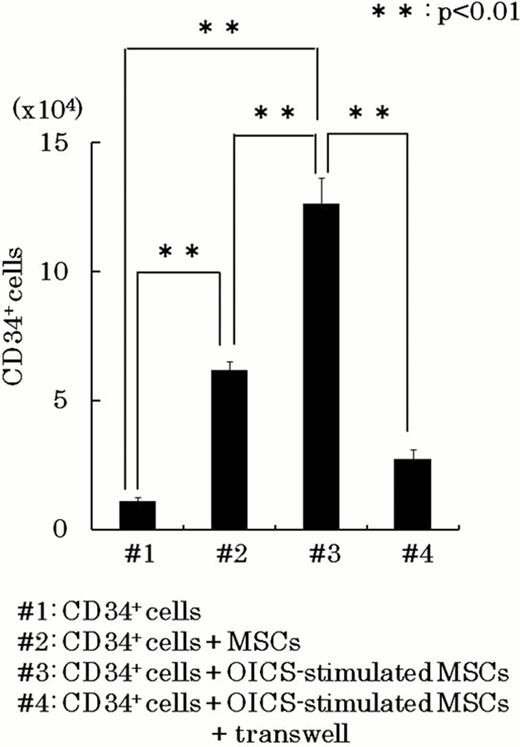
The osteogenesis-induction was achieved by treating human MSCs with ascorbic acid, dexamethasone, and potassium dihydrogen phosphate (osteogenesis-inducing cocktails: OICS). When human MSCs were osteogenesis-induced by OICS for 3 weeks, MSCs differentiated into mature osteoblasts with abundant calcium accumulation assessed by Alizarin red S staining. However, when human MSCs were treated with OICS for short periods, they did not show apparent calcium accumulation but expressed early-stage osteogenic marker, osterix, and maintain differentiation capability to adipocytes. Intriguingly, purified CD34+ hematopoietic cells (5.0×103 cells/dish) were expanded to the number of 12.6±1.01×104 (#3 of the figure) in 10 day co-cultures with the osterix positive osteogenesis-induced cells in StemSpan Medium (StemCell Technologies) supplemented with 100ng/mL SCF, 100ng/mL Flt-3 ligand, 50ng/mL TPO, and 20ng/mL IL-3. As a control, CD34+ hematopoietic cells was expanded to the number of 6.2±0.4×104 (#2 of the figure) in co-cultures of unstimulated MSCs. Moreover, although the most of the hematopoietic cells expanded on unstimulated MSCs showed an immature blast-like morphology, the hematopoietic cells expanded in the co-cultures with OICS-stimulated MSCs showed a tendency to differentiate into the mature hematopoietic cells, which was supported by the expression of glycophorin-A and CD14 on the hematopoietic cells by FACS analysis.
When purified CD34+ hematopoietic cells were co-cultured with OICS-stimulated MSCs in the transwell, the number of expanded CD34+ hematopoietic cells was decreased to 21.7% (#4 of the figure). In contrast, there was no apparent difference in the expression of differentiation markers in the expanded hematopoietic cells between the co-cultures in the presence and in the absence of transwell. Therefore, cell-cell interactions through surface membrane molecules were involved in CD34+ hematopoietic cells expansion mediated by OICS-stimulated MSCs, and soluble factors were mainly involved in the enhancement of hematopoietic differentiation. Real-time PCR analysis showed that the expression of CXCL12 and LIF was reduced in OICS-stimulated MSCs.
Given that osteogenic stimulation of MSCs by OICS enhances the expansion and differentiation of CD34+ hematopoietic cells in vitro, we tested the possibility of in vivo administration of OICS to mice receiving bone marrow transplantation after myeloablative conditioning for obtaining quick hematopoietic recover. Lethally irradiated (9Gy) C57BL/6 mice were injected with OICS on day 1–7 after receiving total bone marrow transplantation. The number of leukocytes was decreased to bottom level around 7 days after transplantation in both OICS-treated and non-treated (control) mice. However, the number of leukocyte showed a rapid increase in OICS-treated mice compared with that in control mice. These results suggested that short-term osteogenic stimulation supports the hematopoietic recovery in vivo, probably in part, through acting on MSCs in bone marrow microenvironment.
In conclusion, osteogenesis-induced, osterix positive mesenchymal stem cells have unique capabilities to enhance both expansion of CD34+ hematopoietic cells through surface membrane molecules, and differentiation of CD34+ hematopoietic cells into mature cells through soluble factors. This work suggests a possibility that “pharmacological stimulation” of MSCs could modify the bone marrow microenvironment through enhancement of biological potency of MSCs. Further studies are needed whether this strategy may be applied in the clinical settings.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal