Abstract
Abstract 3431
The greatest problem in cancer therapy is the tendency of cancerous cells to leave a primary tumor and metastasize to different vital organs. Several candidate classes of molecules have been proposed to direct the metastatic process, including chemokines, growth factors, cytokines and bioactive lipids. It is well known that plasma and serum alone possess chemotactic activity, but all the factors responsible for this effect have not been well characterized. This activity was usually assigned to chemokines (e.g., stromal derived factor-1, SDF-1) and growth factors (e.g., hepatocyte growth factor/scatter factor, HGF/SF) present in the serum. However, the concentration of these factors, as measured by ELISA, is negligibly low and does not explain the robust chemotactic responsiveness of tumor cells to serum samples. To our surprise, however, we found that highly diluted (1%) plasma possesses remarkable chemokinetic activity against cancer cell lines, which exceeds by several times the activity of optimal doses of SDF-1 or HGF/SF.
Based on this intriguing observation, our aim was to better characterize this remarkable chemokinetic activity of normal plasma identified in plasma diluted to 1%.
We employed several human cell lines (lung cancer A549 and HTB177, breast cancer HTB26, cervical carcinoma HTB35, and rhabdomyosracomas RH30 and SMS-CTR) and different dilutions of normal human, murine, and bovine plasma and serum in Transwell migration, adhesion, and cell signaling studies. In chemotactic experiments we also employed samples of human interstitial, pleural, and peritoneal cavity fluids. We tested the effect of heat inactivation, protease exposure, dialysis, and molecular filtration on the chemotactic activity of diluted plasma.
We report for the first time that highly diluted (1%) plasma (human, murine, and bovine) possesses remarkable chemotactic activity against malignant tumor cells in Transwell migration assays. This activity rapidly decreases with increasing concentrations of plasma (>5%). We found that in our cell lines, plasma diluted 1:100 activated phosphorylation of MAPKp42/44 and AKT, and the chemotactic response was inhibited by blocking Gai protein signaling by pertussis toxin (PTX) as well as by inhibition of MAPKp42/44 and AKT, which suggests the involvement of a specific GaI protein-coupled receptor. Our initial characterization of this activity revealed that this remarkable factor is sensitive to proteolytic treatment, is not removed from plasma by dialysis, and is temperature-sensitive, which collectively indicates a polypeptide structure. Electrophoretic studies revealed that this factor possesses a molecular weight between 100 and 50 kD. A similar effect has been observed with serum diluted 1:100, though in contrast with plasma, this chemotactic responsiveness was maintained at higher concentrations. This difference between plasma and serum in responsiveness to dilutions shifted our attention to the possible involvement of an inhibitory effect of fibrinogen. To address the hypothesis that this factor is neutralized or transported by fibrinogen and released from fibrinogen during plasma dilution, we removed fibrinogen from plasma, and, in the reverse experiment, added fibrinogen to serum, expecting that it would quench this factor. As expected, while removal of fibrinogen from plasma increased the chemotactic activity of more concentrated plasma, the addition of fibrinogen to serum inhibited the chemotactic activity of serum. Finally, we observed a very similar phenomenon for diluted fluids harvested from peritoneum, pleura, and intestinal tissue.
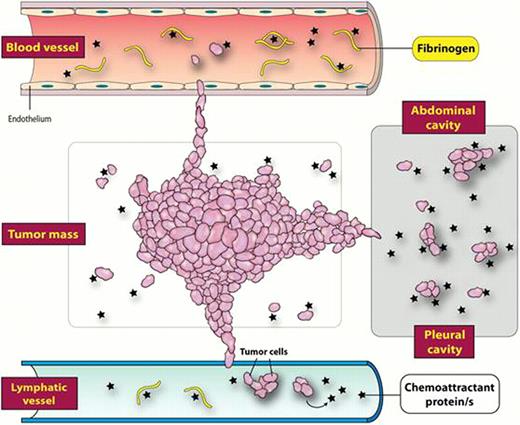
Our data indicate that normal plasma contains protein that direct egress of cancer cells from the tumor and are responsible for metastasis of these cells Figure . This factor (shown by asterices), which is several times more potent that known chemokines or chemotactic growth factors, is neutralized in the presence of the fibrinogen (shown by rods), which may explain the preference of tumor cells to migrate into the lymphatic system and body cavities, where the concentrations of fibrinogen are low. Our signaling studies revealed also that this factor acts through a Gai protein-coupled receptor. We are currently purifying and identifying this protein by employing mass spectophotometry.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal