Abstract
Abstract 3402
Although blood of patients with hematological disorders is known to contain procoagulant material (activated clotting factors, tissue factor, microparticles), its functional significance is poorly understood. Using an in vitro experimental model of immobilized tissue factor-initiated clot growth in platelet-free plasma, we observed formation of activation-surface-independent isolated “spontaneous” clots (SC) throughout the plasma volume in patients with various disorders. The aim of this work was to identify the mechanisms of SC formation.
Platelet-free plasma was prepared from citrated whole blood collected by venipuncture. It was supplemented with corn trypsin inhibitor (CTI), recalcified, placed into a thin flat chamber and brought into contact with a surface covered by immobilized tissue factor. Fibrin clot formation as a function of space and time was determined by imaging light scattering. with a CCD camera1 in a thrombodynamics analyzer device (Hemacore LLC, Moscow, Russia). Flow cytometry was used to monitor annexin V-labeled microparticles and platelets in plasma.
SC were reproducibly observed in plasmas of patients with various disoders including: 4 of 32 patients with ischemic cardiomyopathy, 11 of 29 patients with lymphogranulomatosis during chemotherary, 2 of 5 patients with acute leukemia. SC also appeared in 2 of 36 healthy donor plasmas.
To determine contribution of intrinsic and extrinsic coagulation activation pathways, we blocked them with corn trypsin inhibitor (CTI) and active site-inhibited recombinant factor VIIa, respectively. Tissue factor-dependent activation was essential in 1 of 10 patients tested in this way. CTI inhibited SC in all samples, but never completely (Fig. 1).
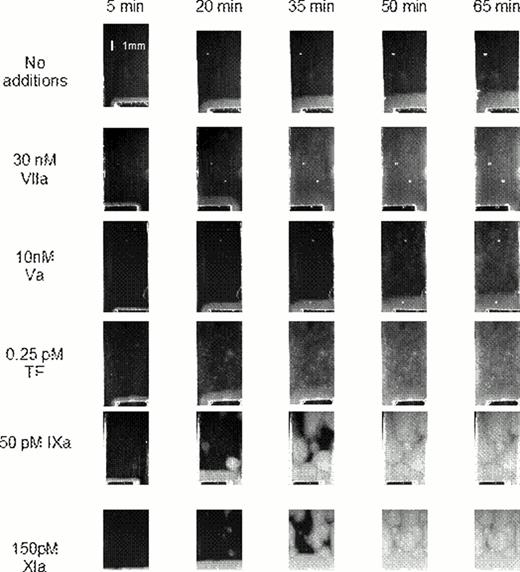
To test whether circulating active factors can induce SC, we supplemented normal plasma with factors Va, VIIa, IXa, or XIa known to have long lifetimes (more than 30 min). Addition of factors Va and VIIa led to uniform coagulation thoughout the plasma volume, which did not resemble typical SC patterns observed in the patient plasma (Fig. 2). In contrast, factors IXa and XIa induced isolated clotting centers very similar to those observed in the clinical samples.
SC both in patient plasma and in normal plasma supplemented with factors IXa and XIa disappeared after centrifugation for 30 min at 16000 g, indicating also a contribution of microparticles. This was confirmed by inductions of SC in the bottom layers of normal plasma subjected to identical centrifugation. After phospholipid supplementation, SC reappeared in normal plasma supplemented with factors IXa or XIa, as well as in 3 out of 11 patient plasmas. Flow cytometry revealed an increase in procoagulant microparticles in patient plasmas with SC.
Here we report observation of a SC phenomenon in vitro in plasma of hematology patients. Although its mechanisms are likely to be different in different illnesses, our data suggest that combination of circulating active factors (specifically, factors IXa and XIa) with circulating procoagulant microparticles is the predominant one. Possible association between the presence of spontaneous clots and clinical manifestations require further study, but prevalence of this phenomenon in the patient plasma indicates possible applicability in diagnostics and.
Contribution of the intrinsic and extrinsic pathways to SC formation.
(A) Typical clot growth images in plasmas of patients, either untreated, or supplemented with VIIai (50 nM), or with CTI (200 μg/ml), or both. Note formation of numerous fibrin clots independent of the TF-iduced thrombus growth (on the bottom of each image). (B) Spontaneous clotting times for ten different patients as determined in the four experimental designs shown in panel A. In most patients, the role of contact pathway is the predominant one.
Contribution of the intrinsic and extrinsic pathways to SC formation.
(A) Typical clot growth images in plasmas of patients, either untreated, or supplemented with VIIai (50 nM), or with CTI (200 μg/ml), or both. Note formation of numerous fibrin clots independent of the TF-iduced thrombus growth (on the bottom of each image). (B) Spontaneous clotting times for ten different patients as determined in the four experimental designs shown in panel A. In most patients, the role of contact pathway is the predominant one.
Contibution of circulating active factors to the SC formation.
Platelet-free plasma was supplemented with indicated coagulation factors, and the pattern of spatial clotting induced by immobilized TF was nalyzed. Images at different timepoints are shown.
Contibution of circulating active factors to the SC formation.
Platelet-free plasma was supplemented with indicated coagulation factors, and the pattern of spatial clotting induced by immobilized TF was nalyzed. Images at different timepoints are shown.
Lipets:HemaCore LLC: Employment. Ataullakhanov:HemaCore LLC: Employment, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties. Panteleev:HemaCore LLC: Employment, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties.
References
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal