Abstract
Abstract 3401
Early identification of children with deep venous thrombosis (DVT) of the limb, whoare at heightened risk for post-thrombotic syndrome (PTS), is important in order to evaluate therapeutic interventions aimed at decreasing the risk and severity of PTS.
We sought to evaluate prognostic factors for PTS in children following DVT of the limbs.
In this bi-institutional mixed cohort study with prospective ascertainment of PTS using a validated pediatric instrument, we collected data on patient/thrombus characteristics, thrombophilia testing results, and outcomes in children (<21 years at event) diagnosed with acute limb DVT at Rady Children's Hospital of San Diego and Children's Hospital Colorado.
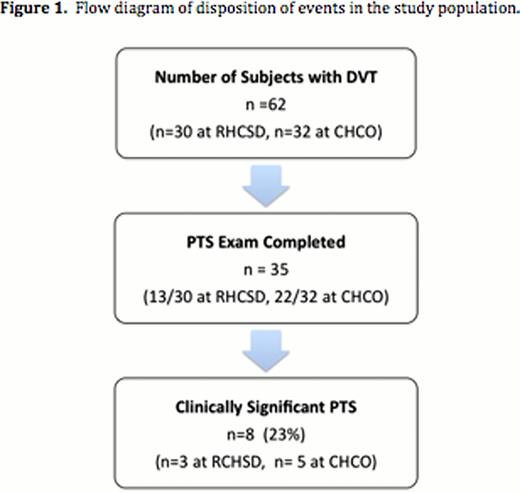
Median age at presentation was 13 years (range, 0–18 years). Cumulative incidence (i.e., risk of PTS) was 23%, at a median follow-up duration of 33 months (range: 13.2–65 months). The presence of a lupus anticoagulant by dilute Russell Viper venom time (dRVVT) testing within two weeks of DVT diagnosis was associated with markedly increased odds of developing clinically-significant PTS (OR 16.8, 95%CI (1.60–176.2); P=0.02). The presence of an infectious or inflammatory condition at DVT presentation was neither associated with PTS risk nor dRVVT positivity.
An acutely positive dRVVT following diagnosis of limb DVT appears to be a significant prognostic factor for development of clinically significant PTS in children. Larger collaborative cohort studies are required to substantiate these findings, evaluate other prognostic factors, and determine whether the present association is modulated by persistent dRVVT positivity or beta-2-glycoprotein-I dependence.
Patient Characteristics
| Variable . | DVT events n=35 . | Clinically Significant PTS n=8 . | No Clinically Significant PTS n=27 . | P value . |
|---|---|---|---|---|
| Age (year) | ||||
| Median | 13 | 15.5 | 10 | 0.06 |
| Range | 0.2–18 | 0.6–17 | 0.2–18 | |
| Gender (%) | 0.41 | |||
| Male | 22 (63) | 4 (50) | 18 (67) | |
| Female | 13 (37) | 4 (50) | 9 (33) | |
| BMI % | ||||
| Median | 73.1 | 68.75 | 76.45 | 0.48 |
| Range | 0.1–99.8 | 0.1–96 | 18.9–99.8 | |
| Clinical Characteristics (%) | ||||
| Central Line Associatedà | 14 (40) | 2 (25) | 12 (44) | 0.43 |
| Infection/Inflammatory ConditionÆ | 14 (40) | 3 (38) | 11 (41) | 1.00 |
| Laboratory Parameters | ||||
| FV Leiden Heterozygote – (%) | 7 (20) | 2 (25) | 5 (19) | 1.00 |
| FV Leiden Homozygote – (%) | 1 (3) | 1 (13) | 0 (0) | 0.23 |
| FII G20210A heterozygote– no. tested (%) | 0/34 (0) | 0/8 (0) | 0/26 (0) | 1.00 |
| Moderate/Severe protein C, protein S, or AT deficiency – no. tested (%) | 3/30 (10) | 0/7 (0) | 3/23 (13) | 1.00 |
| FVIII Level (IU/dL) | (n=19) | (n =4) | (n=15) | |
| Median (n =23) | 221A | 226.5 | 208 | 0.55 |
| Range | 99.9–413.7 | 215.9–295.5 | 99.9–413.7 | |
| dRVVT – no. positive/tested (%) | 11/26 (42) | 6/7 (86) | 5/19 (26) | 0.02 |
| D-dimer > 500 ng/mL (%) | 20/26 (77) | 6/6 (100) | 14/20 (70) | 0.28 |
| Veno-Occlusion at Diagnosis (%) | 23 (66) | 8 (100) | 15 (56) | 0.03 |
| Veno-Occlusion 3 months after diagnosis (%) | 7 (20) | 2 (25) | 5 (19) | 0.65 |
| Recurrent VTE (%) | 7 (20) | 2 (25) | 5 (19) | 0.65 |
| Follow-Up (months) | ||||
| Median | 33 | 40 | 30 | 0.27 |
| Range | 13.2–65 | 19–60 | 13–65 |
| Variable . | DVT events n=35 . | Clinically Significant PTS n=8 . | No Clinically Significant PTS n=27 . | P value . |
|---|---|---|---|---|
| Age (year) | ||||
| Median | 13 | 15.5 | 10 | 0.06 |
| Range | 0.2–18 | 0.6–17 | 0.2–18 | |
| Gender (%) | 0.41 | |||
| Male | 22 (63) | 4 (50) | 18 (67) | |
| Female | 13 (37) | 4 (50) | 9 (33) | |
| BMI % | ||||
| Median | 73.1 | 68.75 | 76.45 | 0.48 |
| Range | 0.1–99.8 | 0.1–96 | 18.9–99.8 | |
| Clinical Characteristics (%) | ||||
| Central Line Associatedà | 14 (40) | 2 (25) | 12 (44) | 0.43 |
| Infection/Inflammatory ConditionÆ | 14 (40) | 3 (38) | 11 (41) | 1.00 |
| Laboratory Parameters | ||||
| FV Leiden Heterozygote – (%) | 7 (20) | 2 (25) | 5 (19) | 1.00 |
| FV Leiden Homozygote – (%) | 1 (3) | 1 (13) | 0 (0) | 0.23 |
| FII G20210A heterozygote– no. tested (%) | 0/34 (0) | 0/8 (0) | 0/26 (0) | 1.00 |
| Moderate/Severe protein C, protein S, or AT deficiency – no. tested (%) | 3/30 (10) | 0/7 (0) | 3/23 (13) | 1.00 |
| FVIII Level (IU/dL) | (n=19) | (n =4) | (n=15) | |
| Median (n =23) | 221A | 226.5 | 208 | 0.55 |
| Range | 99.9–413.7 | 215.9–295.5 | 99.9–413.7 | |
| dRVVT – no. positive/tested (%) | 11/26 (42) | 6/7 (86) | 5/19 (26) | 0.02 |
| D-dimer > 500 ng/mL (%) | 20/26 (77) | 6/6 (100) | 14/20 (70) | 0.28 |
| Veno-Occlusion at Diagnosis (%) | 23 (66) | 8 (100) | 15 (56) | 0.03 |
| Veno-Occlusion 3 months after diagnosis (%) | 7 (20) | 2 (25) | 5 (19) | 0.65 |
| Recurrent VTE (%) | 7 (20) | 2 (25) | 5 (19) | 0.65 |
| Follow-Up (months) | ||||
| Median | 33 | 40 | 30 | 0.27 |
| Range | 13.2–65 | 19–60 | 13–65 |
Central line at time of VTE or within the preceding 30 days.
Inflammatory/Infectious Conditions include acute infection (sepsis n=2, influenza n=2, pneumonia n=2, viral encephalitis n=1, osteomyelitis n=1, necrotizing enterocolitis n=1, CNS tuberculosis n=1), inflammatory bowel disease n=1, hemolytic uremic syndrome n=2, diabetic ketoacidosis n=1, systemic lupus erythematosus n= 1, anti-NMDA receptor encephalitis n= 1, malignancy n=1 (Several subjects with > 1 disorder).
Univariate Logistic Regression Analysis of Prognostic Factors for Clinically-Significant PTS* Following Diagnosis of VTE
| Prognostic Factor . | Univariate Odds Ratio (95% CI) . | P value . |
|---|---|---|
| Age | 1.15 (0.96–1.38) | 0.12 |
| BMI | 0.99 (0.96–1.02) | 0.38 |
| Gender | 0.50 (0.10–2.48) | 0.40 |
| Central Line | 0.42 (0.07–2.45) | 0.33 |
| Veno-Occlusion at Diagnosis | 4.15 (0.42–40.6) | 0.22 |
| Proximal DVT | 0.88 (0.08–9.79) | 0.91 |
| Inflammatory or Infectious condition | 0.87 (0.17–4.43) | 0.87 |
| FVIII | 1.00 (0.99–1.02) | 0.78 |
| dRVVT | 16.8 (1.60–176.2) | 0.02 |
| D-dimer > 500 ng/mL | 2.14 (0.2–22.5) | 0.53 |
| Inherited thrombophilia# | 1.72 (0.32–9.10) | 0.53 |
| Prognostic Factor . | Univariate Odds Ratio (95% CI) . | P value . |
|---|---|---|
| Age | 1.15 (0.96–1.38) | 0.12 |
| BMI | 0.99 (0.96–1.02) | 0.38 |
| Gender | 0.50 (0.10–2.48) | 0.40 |
| Central Line | 0.42 (0.07–2.45) | 0.33 |
| Veno-Occlusion at Diagnosis | 4.15 (0.42–40.6) | 0.22 |
| Proximal DVT | 0.88 (0.08–9.79) | 0.91 |
| Inflammatory or Infectious condition | 0.87 (0.17–4.43) | 0.87 |
| FVIII | 1.00 (0.99–1.02) | 0.78 |
| dRVVT | 16.8 (1.60–176.2) | 0.02 |
| D-dimer > 500 ng/mL | 2.14 (0.2–22.5) | 0.53 |
| Inherited thrombophilia# | 1.72 (0.32–9.10) | 0.53 |
Score > 1 in both physical examination and functional limitation categories, according to the Manco-Johnson instrument, a standardized PTS assessment tool.
Presence of one or more of the following: factor V Leiden polymorphism, factor II G20210A polymorphism, or plasma protein C activity < 40%; plasmas free protein S antigen level <40%, or plasma antithrombin activity < 60% after 6 months of age.
Goldenberg:Eisai Inc: Research Funding; Pfizer Inc: Membership on an entity's Board of Directors or advisory committees; CPC Clinical Research: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal