Abstract
Abstract 3388
Primary Immune Thrombocytopenia (ITP) is an autoimmune disorder characterized by a low platelet count below 100 × 109/L and an exclusion of secondary causes such as bacterial infections or hematological malignancies. Fc-gamma-receptors (FcγRs) are involved in the clearance of immune complexes, the antibody-dependent cellular cytotoxicity, the secretion of mediators and indirectly the regulation of B cell proliferation and differentiation. The investigational product SM101 represents the non-glycosylated C-terminally engineered human soluble FcγRIIB. SM101 has been investigated successfully in autoimmune models, toxicology studies and safety clinical trials in healthy subjects and is currently being investigated in phase II clinical studies in patients with ITP and Systemic Lupus Erythematosus (SLE). Recently, the dose-escalation part of the phase Ib/IIa clinical trial in ITP was completed.
The primary objective was to evaluate the safety and tolerability of SM101 at increasing dose levels in patients with chronic ITP. Secondary objectives included efficacy in terms of platelet count and pharmacokinetic (PK) evaluation of SM101.
The Ib part of the clinical trial was designed as a randomized, double-blind, placebo-controlled, multi-center dose escalation study in 36 ITP patients. Each dosing cohort consisted of 4 verum and 2 placebo subjects. Major inclusion criteria included a diagnosis of chronic ITP for at least 6 months and a platelet count of less than 30 × 109/L at baseline. Concomitant corticosteroids were allowed at constant doses during the study. Eligible patients were dosed on day 1 with a 5 week safety period, followed up by the intended treatment cycle of 4 weekly infusions between day 35 and 56 and a final follow-up of 3 months. An independent Data Safety Monitoring Board was in place to review safety data after each dose cohort.
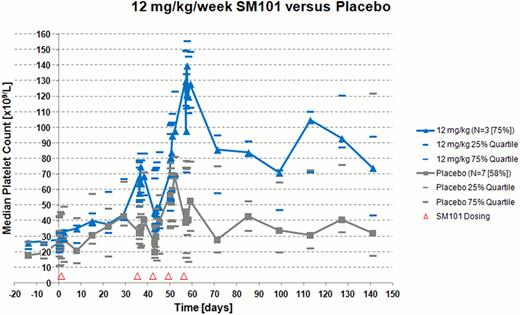
In total, 36 ITP patients in 6 dose groups received 5 infusions of 0.3 to 12.0 mg/kg/week SM101or placebo. 56% of the patients were females, 39% of the patients were splenectomized and 39% received concomitant corticosteroids prior to randomization. In the placebo group, 42% received rescue medication compared to 0 to 25% in the higher dose groups with SM101. No dose-limiting toxicity (DLT) occurred and no serious adverse event (SAE) was observed for patients treated with SM101. SM101 plasma levels increased proportionally with dose and the mean t1/2 across all dose groups was 42.7 h with a range of 14.0 to 99.9 h. In terms of immunogenicity, no anti-drug-antibodies against SM101 were observed. For patients without ITP rescue therapy, the highest dose group with 12 mg/kg/week SM101 showed a sustained median platelet response over 50 × 109/L in comparison to placebo after one treatment cycle with 4 infusions (Figure 1). This response persisted during the 3 months follow-up period with a median platelet count of approximately 70 × 109/L at the end of the clinical trial. The platelet response in the 12 mg/kg/week group started approximately 20 days after the beginning of the treatment cycle.
- To the author's knowledge, this is the first time that a soluble Fcγ receptor showed efficacy in a clinical trial
- In the 12 mg/kg/week group, SM101 showed a clear platelet response in patients with ITP
- The duration of the platelet response persisted for at least 3 months after the last administration of SM101
- Multiple doses of SM101 up to 12 mg/kg/week were well tolerated with no DLT or SAE
- Further clinical trials with SM101 in ITP to support the current findings are ongoing.
Tillmanns:SuppreMol GmbH: Employment. Sondermann:SuppreMol GmbH: Employment. Buckel:SuppreMol GmbH: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal