Abstract
Abstract 3382
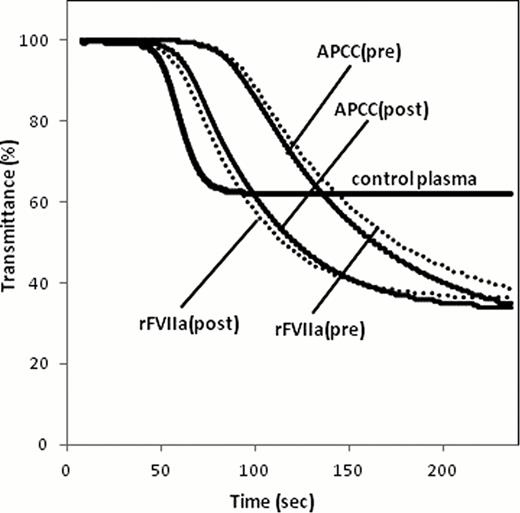
There is no optimal assay to monitor the hemostatic effect of bypassing therapy for hemophilia A patients (HA) with inhibitor. Clot waveform analysis (CWA) is a convenient method of assessing global clotting function based on the continuous monitoring of light transmittance or absorbance during routine coagulation tests such as aPTT. We attempted to optimize the aPTT based CWA for hemostatic monitoring during the bypassing therapy in patients of HA with inhibitor. An automated laboratory system, MDA-II® (Trinity Biotech) was used for CWA. Clot waveform was plotted from transmittance change during the clot formation. By the first and second derivation of the waveform, parameters such as clotting time, the maximum velocity (|min1|) and acceleration (|min2|) were calculated. Three trigger reagents were used; [R1] ellagic acid (E) and phospholipid (PL) that is used as an aPTT reagent, [R2] tissue factor (TF, Innovin®) and PL as a 17,000-diluted PT reagent, [R3] the mixed condition of E, TF and PL, based on our recent report on thrombin generation test optimized for HA (Matsumoto, IJH, 2009). Results were expressed as a percentage (% of control) relative to control pooled plasma. The great difference in all parameters between factor (F)VIII-deficient and control plasma resulted in R1>R3>>R2 in order (|min2| in R1, R2, R3 were 13, 119, 21% of control, respectively). Since significant difference between both plasma was not observed, we excluded R2. By the addition of clinically therapeutic concentration of recombinant FVIIa (rFVIIa, NovoSeven®) (25 nM), parameters in R3 were more improved than R1 (|min2| in R1, R3 were 18, 47% of control, respectively). Similar results were observed by the addition of activated prothrombin complex concentrates (APCC, FEIBA®) (1 U/ml) (|min2| in R1, R3 was 32, 71% of control, respectively). According to these in vitro experiments, R3 (E/TF/PL) was regarded as most optimal reagent. Next, in order to confirm the usefulness of the R3-CWA system in vivo, six patients with HA with inhibitor to whom rFVIIa (n=6, 37 (12–59) BU/ml, 105 (91–175) ƒÊg/kg dosage) or APCC (n=3, 25 (12–49) BU/ml, 91 (91–100) U/kg dosage) was administrated, were evaluated. All patients showed the clinical hemostatic efficacy by each bypassing agent. As shown in Table 2 and Fig. 1, the hemostatic effects by bypassing agents were confirmed by improvement in all R3-CWA parameters. APTT based CWA system should be promising method for quantitative monitoring during the bypassing therapy with routine automated clotting machine and only with the modified reagents such as well-balanced mixtures of E, TF and PL.
Parameters of CWA using three reagents in FVIII-deficient plasma with bypassing agents in vitro
| Parameter . | Reagent . | ||
|---|---|---|---|
| R1 . | R2 . | R3 . | |
| Clotting time | % of control | ||
| FVIII-def plasma | 372 | 86 | 147 |
| +rFVIIa (25 nM) | 271 | – | 100 |
| +APCC (1 U/ml) | 122 | – | 69 |
| |min1| | % of control | ||
| FVIII-def plasma | 16 | 90 | 33 |
| +rFVIIa (25 nM) | 20 | – | 54 |
| +APCC (1 U/ml) | 27 | – | 69 |
| |min2| | % of control | ||
| FVIII-def plasma | 13 | 119 | 21 |
| +rFVIIa (25 nM) | 18 | – | 47 |
| +APCC (1 U/ml) | 32 | – | 71 |
| Parameter . | Reagent . | ||
|---|---|---|---|
| R1 . | R2 . | R3 . | |
| Clotting time | % of control | ||
| FVIII-def plasma | 372 | 86 | 147 |
| +rFVIIa (25 nM) | 271 | – | 100 |
| +APCC (1 U/ml) | 122 | – | 69 |
| |min1| | % of control | ||
| FVIII-def plasma | 16 | 90 | 33 |
| +rFVIIa (25 nM) | 20 | – | 54 |
| +APCC (1 U/ml) | 27 | – | 69 |
| |min2| | % of control | ||
| FVIII-def plasma | 13 | 119 | 21 |
| +rFVIIa (25 nM) | 18 | – | 47 |
| +APCC (1 U/ml) | 32 | – | 71 |
Values are shown as median of triplicate.
Effect of bypassing agents in vivo on parameters of R3-triggered CWA
| Parameter . | rFVIIa (n=6) . | APCC (n=3) . | ||
|---|---|---|---|---|
| Pre . | Post . | Pre . | Post . | |
| % of control | % of control | |||
| Clotting time | 202 | 103 | 135 | 99 |
| (129–268) | (91–122) | (127–159) | (89–104) | |
| |min1| | 37 | 56 | 41 | 60 |
| (19–48) | (40–78) | (30–42) | (46–75) | |
| |min2| | 19 | 53 | 23 | 41 |
| (13–32) | (44–75) | (16–24) | (34–60) | |
| Parameter . | rFVIIa (n=6) . | APCC (n=3) . | ||
|---|---|---|---|---|
| Pre . | Post . | Pre . | Post . | |
| % of control | % of control | |||
| Clotting time | 202 | 103 | 135 | 99 |
| (129–268) | (91–122) | (127–159) | (89–104) | |
| |min1| | 37 | 56 | 41 | 60 |
| (19–48) | (40–78) | (30–42) | (46–75) | |
| |min2| | 19 | 53 | 23 | 41 |
| (13–32) | (44–75) | (16–24) | (34–60) | |
Values are shown as median (range).
Representative data of R3-triggered CWA in a case of HA with inhibitor on bypassing therapy using rFVIIa (dotted line) or APCC (solid line).
Representative data of R3-triggered CWA in a case of HA with inhibitor on bypassing therapy using rFVIIa (dotted line) or APCC (solid line).
Shima:Chugai Pharmaceutical Co., Ltd.: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal