Abstract
Abstract 3332
Corticosteroids are the initial standard therapy for primary immune thrombocytopenia (ITP). A short course of high-dose dexamethasone as initial therapy is an alternative to prednisone with high initial responses, but about half of the patients relapse. Low dose rituximab has been used in the treatment of relapsed ITP, showing a similar activity to the standard dose. The aim of this prospective study was to evaluate the efficacy, safety and response duration of low-dose rituximab plus short pulses of high-dose dexamethasone as front-line therapy in newly diagnosed ITP adults.
Patients ≥18 years old with newly diagnosed received dexamethasone, 40 mg/day/i.v. for 4 consecutive days (+1, +2, +3, +4), rituximab was administered at a fixed dose of 100 mg as an i.v. infusion weekly for 4 consecutive doses (days +1, +8+, +15 and +22). The use of rescue therapy was allowed, using the same schedule of dexamethasone, before day 30, but only if the platelet count was < 20×109/L.
A complete blood cell count was performed at enrollment, weekly for the first 28 days, monthly until month 6 and then every 3 months. The degree of response was defined as follows: a complete response (CR) was a platelet count ≥ 100 × 109/L; a complete sustained response (CSR) was considered if it was maintained for six months. Partial response (PR) was defined as a platelet level between 50 and 100 × 109/L; an overall response (OR) was defined as partial or complete response. Patients with a platelet count <30 × 109/L were considered non-responders (NR). Time-to-response (TTR) and time-to-complete response (TCR) were also assessed, as well as the safety profile and side effects incidence according to the National Cancer Institute Common Toxicity Criteria version 3.0.
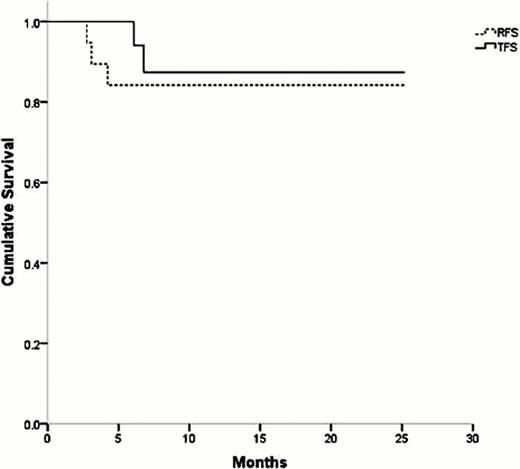
Twenty-one consecutive patients were enrolled from December 2009 to December 2011 (17 women and 4 men); median age was 51 years (range, 18–82). The median platelet count at baseline was 5.19 × 109/L (range, 0.3–24.2 × 109/L). Patients were followed for a median of 12 months (range, 1–25). At day +28, sixteen patients (76.2%) had CR, three patients had PR (14.3%), with the OR being 90.5%. At month six, 16 of 21 patients (76.2%) had achieved CSR. Seven patients received a second course of dexamethasone (one at day +8 and six at day+15) (Table 1). The median duration of response was 12 months (range, 7–25 months). The median time to reach TTR and TCR was 8 days (range, 4–28). Partial response was achieved in two patients who were further treated with danazol and prednisone, being subsequently splenectomized. The relapse rate was 15.8%. The 6- and 12- month cumulative RFS were both 84%; the 6- and 12-month cumulative TFS probabilities were 94% and 87%, respectively (Fig. 1).
Overall, combination therapy in our group was well tolerated with a lower incidence of adverse effects during infusion in this trial according to the data reported in previous studies.
The combination of low-dose rituximab and high-dose dexamethasone as front-line therapy for adults with ITP was effective and safe with a high OR rate and a low incidence of relapse. These data need to be confirmed in a prospective randomized clinical trial including a sample size with enough power to reach statistically significant conclusions.
Salient features and response for 21 immune thrombocytopenic purpura (ITP) patients treated with high-dose dexamethasone and rituximab as front-line therapy
| Patient no. . | Age, y/Sex . | Basal platelets × 109/L . | Best response . | Response at day 28 . | Time to PR/CR, wk . | Duration of response, months . | Current status . | Follow up, months . | Additional Treatment . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F/49 | 5.7 | CR | CR | 1/1 | 15 | CSR | 15 | |
| 2 | M/54 | 18.4 | CR | CR | 1/1 | 23 | CSR | 23 | |
| 3 | F/54 | 15 | CR | CR | 1/3 | 25 | CSR | 25 | Dex |
| 4 | F/32 | 8 | CR | CR | 1/1 | 15 | CSR | 15 | |
| 5 | F/56 | 2.5 | CR | CR | 2/2 | 21 | CSR | 21 | Dex |
| 6 | F/64 | 18 | PR | PR | 1/− | – | PUR | 21 | P, D, S |
| 7 | F/18 | 17.8 | PR | PR | 1/1 | – | PUR | 11 | P, D, S |
| 8 | F/73 | 4.9 | CR | PR | 1/1 | – | CUR | 18 | D |
| 9 | F/49 | 4 | CR | CR | 1/1 | 20 | CSR | 20 | |
| 10 | M/19 | 0.9 | NR | NR | −/− | – | NR | 5 | |
| 11 | M/58 | 8 | CR | CR | 3/3 | 12 | CSR | 12 | Dex |
| 12 | F/51 | 7 | CR | CR | 2/2 | 9 | CSR | 9 | |
| 13 | F/58 | 3 | CR | CR | 1/1 | 12 | CSR | 12 | |
| 14 | F/80 | 1.6 | CR | CR | 3/3 | 12 | CSR | 12 | Dex |
| 15 | F/29 | 24.2 | CR | CR | 1/1 | 6 | CSR | 6 | |
| 16 | F/62 | 5 | CR | CR | 1/4 | 12 | CSR | 12 | Dex |
| 17 | F/82 | 0.3 | NR | NR | −/− | – | NR | 1 | |
| 18 | M/41 | 0.4 | CR | CR | 1/1 | 7 | CSR | 7 | Dex |
| 19 | F/40 | 5.38 | CR | CR | 1/1 | 6 | CSR | 6 | |
| 20 | F/21 | 3 | CR | CR | 3/3 | 12 | CSR | 12 | Dex |
| 21 | F/33 | 2 | CR | CR | 4/4 | 6 | CSR | 6 |
| Patient no. . | Age, y/Sex . | Basal platelets × 109/L . | Best response . | Response at day 28 . | Time to PR/CR, wk . | Duration of response, months . | Current status . | Follow up, months . | Additional Treatment . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F/49 | 5.7 | CR | CR | 1/1 | 15 | CSR | 15 | |
| 2 | M/54 | 18.4 | CR | CR | 1/1 | 23 | CSR | 23 | |
| 3 | F/54 | 15 | CR | CR | 1/3 | 25 | CSR | 25 | Dex |
| 4 | F/32 | 8 | CR | CR | 1/1 | 15 | CSR | 15 | |
| 5 | F/56 | 2.5 | CR | CR | 2/2 | 21 | CSR | 21 | Dex |
| 6 | F/64 | 18 | PR | PR | 1/− | – | PUR | 21 | P, D, S |
| 7 | F/18 | 17.8 | PR | PR | 1/1 | – | PUR | 11 | P, D, S |
| 8 | F/73 | 4.9 | CR | PR | 1/1 | – | CUR | 18 | D |
| 9 | F/49 | 4 | CR | CR | 1/1 | 20 | CSR | 20 | |
| 10 | M/19 | 0.9 | NR | NR | −/− | – | NR | 5 | |
| 11 | M/58 | 8 | CR | CR | 3/3 | 12 | CSR | 12 | Dex |
| 12 | F/51 | 7 | CR | CR | 2/2 | 9 | CSR | 9 | |
| 13 | F/58 | 3 | CR | CR | 1/1 | 12 | CSR | 12 | |
| 14 | F/80 | 1.6 | CR | CR | 3/3 | 12 | CSR | 12 | Dex |
| 15 | F/29 | 24.2 | CR | CR | 1/1 | 6 | CSR | 6 | |
| 16 | F/62 | 5 | CR | CR | 1/4 | 12 | CSR | 12 | Dex |
| 17 | F/82 | 0.3 | NR | NR | −/− | – | NR | 1 | |
| 18 | M/41 | 0.4 | CR | CR | 1/1 | 7 | CSR | 7 | Dex |
| 19 | F/40 | 5.38 | CR | CR | 1/1 | 6 | CSR | 6 | |
| 20 | F/21 | 3 | CR | CR | 3/3 | 12 | CSR | 12 | Dex |
| 21 | F/33 | 2 | CR | CR | 4/4 | 6 | CSR | 6 |
PR, partial response; CR, complete response; CSR, complete sustained response; NR, no response; PUR partial unsustained response; CUR, complete unsustained response; P, prednisone; D, danazol; S, splenectomy; Dex, dexamethasone.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal