Abstract
Abstract  3204
3204
For the majority with iron deficient anemia (IDA) a standard full repletion course of IV iron is 1 g. Iron sucrose and ferric gluconate require 4–10 administrations of 100–300mg to deliver a full course. We have employed TDI of 1 g LMW iron dextran, initially over 4 hours and now over 1 hour, in over two thousand patients with good success. No serious adverse events (SAEs) were observed. We sought to explore the potential for further convenience for patients and physicians alike afforded by administering 1.02 g of ferumoxytol (FER) as a 15 min infusion in a single setting instead of the standard regimen of 2 × 510mg injections separated by approximately 1 week. Herein we report the efficacy and safety of such an approach.
60 consecutive patients with IDA (hemoglobin [Hgb] <11 g/dL, transferrin saturation [TSAT] ≤19%, ferritin <100 ng/mL) and a history of intolerance of, or inadequate response to oral iron, received 1020 mg (34 mL) of FER diluted in 100 mL normal saline via infusion pump over 15 minutes. Vital signs were measured for 1 hour post-dose and adverse events (AEs) were collected via phone call 1, 2, and 7 days post-dose, and at clinic visits at 4 and 8 weeks post-dose. Efficacy assessments (Hgb, TSAT, ferritin, red blood cell distribution width [RDW]) occurred at 4 and 8 weeks post-dose. The primary endpoint was safety and tolerability (rate of AEs), while secondary efficacy endpoints included mean change in Hgb level, TSAT and RDW from Baseline to Week 4 and 8.
The mean age was 53.4 (24 – 89) years. The most common underlying cause for IDA was menorrhagia, followed by gastric bypass. No serious AEs occurred, and all but 2 received the planned dose. One subject complained of cough and flushing after initiation of the infusion and requested discontinuation with symptoms abating within 2 minutes without treatment. A second subject developed cough and pruritus, was treated with diphenhydramine and steroids and the symptoms resolved in minutes. Tryptase, a mast cell degranulation marker, was normal in both subjects. Twenty-six of 60 patients reported AEs. Thirteen of the AEs were mild and transient reactions associated with the infusion, none of which received therapy with the exception of the one previously described. All symptoms resolved completely within minutes. Thirteen reported mild self limited arthralgias, myalgias and/or headache within 24–48 hours after treatment.
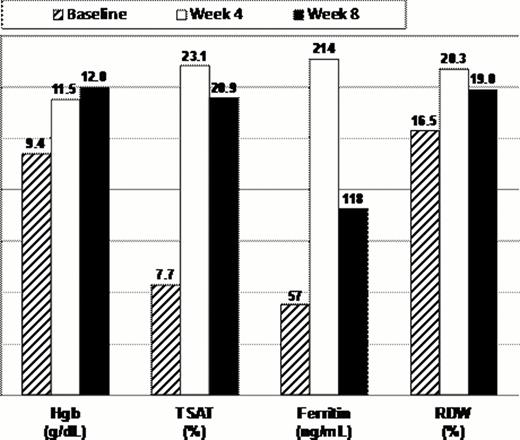
At baseline, the mean Hgb was 9.4 g/dL, TSAT 7.7%, ferritin 57 ng/mL and RDW 16.5%. At 4 weeks, the mean Hgb increase was 2.1 g/dL, and by 8 weeks, the mean increase in Hgb was 2.6 g/dL. The peak increase in TSAT occurred at Week 4, increasing from 15.2 to 23.1%, compared to an increase of 13.2 to 20.9% at Week 8. Similarly, ferritin was maximal at week 4 (214 ng/mL) and had decreased by week 8 to 118 ng/mL. In approximately 90% of subjects, the RDW was increased at both four and 8 weeks post-ferumoxytol administration, consistent with reticulocytosis and ongoing hematopoietic response.
| . | Baseline . | Week 4 . | Week 8 . |
|---|---|---|---|
| Hgb | 9.4 ± 1.2 | 11.5 ± 1.1 | 12.0 ± 1.2 |
| TSAT | 7.7 ± 5.1 | 23.1 ± 9.7 | 20.9 ± 13.6 |
| Ferritin | 57.4 ± 187.1 | 213.6 ± 336.7 | 118.4 ± 223.2 |
| RDW | 16.5 ± 1.9 | 20.3 ± 3.6 | 19.0 ± 3.1 |
| . | Baseline . | Week 4 . | Week 8 . |
|---|---|---|---|
| Hgb | 9.4 ± 1.2 | 11.5 ± 1.1 | 12.0 ± 1.2 |
| TSAT | 7.7 ± 5.1 | 23.1 ± 9.7 | 20.9 ± 13.6 |
| Ferritin | 57.4 ± 187.1 | 213.6 ± 336.7 | 118.4 ± 223.2 |
| RDW | 16.5 ± 1.9 | 20.3 ± 3.6 | 19.0 ± 3.1 |
Key Efficacy Parameters Following Total Dose Infusion (1.02g) of Ferumoxytol via IV Infusion
Key Efficacy Parameters Following Total Dose Infusion (1.02g) of Ferumoxytol via IV Infusion
Ferumoxytol, administered as a 1.02g TDI over 15 min was well tolerated with no SAEs in IDA patients with a history of intolerance of, or inadequate response to oral iron. Ferumoxytol also demonstrated excellent efficacy in this population with a diverse group of etiologies for IDA. By 4 weeks, Hgb had increased 2.1 g/dL with a continued further increase to 2.6 g/dL by Week 8. RDWs were elevated at 4 and 8 weeks in 90% of subjects, consistent with an ongoing hematologic response. This study of 60 iron deficient anemia patients demonstrates the safety and ease of administering a complete 1.02g replacement dose of IV iron in 15 minutes with ferumoxytol. If these results are corroborated in other studies, the infusion of 1.02 g of ferumoxytol as a 15 minutes infusion represents an improved method of treating iron deficient anemia.
Off Label Use: 1020 mg of ferumoxytol is off label as the maximum approved dose is 510 mg. Further the drug was administered to patients with a host of conditions associated with iron deficiency and currently is only on label for iron deficiency associated with chronic kidney disease. The purpose is to provide a full replacement dose of iron in 15 minutes, representing a significant improvement in the management of iron deficient anemia. Strauss:AMAG Pharmaceuticals, Inc.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal