Abstract
Chemo-immunotherapy (i.e. rituximab combinations) has clearly had a significant impact on the outcome of all B-cell NHL both in terms of PFS and OS. However in the relapse/refractory setting a large proportion of pts still do very poorly especially in aggressive subtypes including DLBCL and MCL. Salvage therapy followed by HDT-ASCT in relapsed DLBCL remains the standard, though pts with early failures (<1y) and/or prior exposure to rituximab still show dismal results (CORAL data). In MCL the use of HDT-ASCT in the relapse setting is debated given the frequency of chemo-resistance leading to poor results even in second CR. The use of allogeneic transplantation was developed based on observations c/w with a clear GVL effect in NHL as illustrated by pts going into remission after DLI injections. The development of non-myeloablative approaches has allowed expansion of use of allogeneic BMT in relapsed/refractory NHL. We report here one of the largest series (179 consecutive pts) with relapsed/refractory lymphoma focusing on overall survival and outcome predictors.
Utilizing Kaplan-Meier survival and Cox regression methods, we report on the outcome of 179 consecutive pts with relapsed/refractory lymphoma who underwent allogeneic stem cell transplantation at the John Theurer Cancer Center between 1995–2012. The primary study endpoint was overall survival (OS) assessed by chart and SSDI database review. Secondary study endpoints included examination of the association between overall survival and allogeneic stem cell source, donor source, development of GVHD, pre-transplant chemo-sensitivity and prior failure to HDT-ASCT (second transplant). The proportional hazards assumption was met for this analysis.
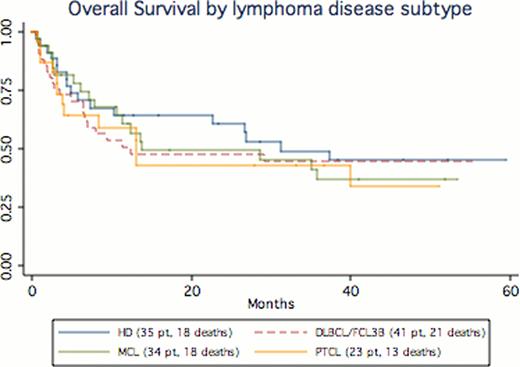
Survival data on 179 pts (median age 48, range 20–71) were analyzed, representing 86 deaths and 5720 total months at risk (median follow up=12.3 months). Baseline characteristics included: ECOG PS (med 1, range 0–2), diagnosis (25% DLBCL, 21% HD, 20% MCL, 13% FCL, 13% PTCL, 8% other), donor source (50% matched SIB, 31% MUD, 19% mismatched MUD), stem cell source (73% PB, 23% BM, 6% Cord) and prior autologous SCT (38%). The median OS for the entire cohort was 31.2 months. OS KM curves by selected aggressive NHL subtypes are represented in Figure 1. We performed COX regression analyses to address outlined secondary endpoints. In univariate analyses statistically significant inferior outcomes were associated with the use of mismatched unrelated donor (HR 1.4, p=.01, Figure 2), bone marrow donor stem cells vs. PBSCT (HR=1.7 p=.04), pre-transplant stable/refractory disease (HR 1.8, p=.03), absence of cGVHD (HR=4.7, p<.001) and presence of acute GVHD (HR 2.8, p=.001). No difference in OS was detected whether pts had undergone allogeneic SCT as a second transplant (med time between auto/allo=20.9 months) following relapse after auto SCT (HR 1.14, CI .75–1.73, p=.5).
This series represents a large cohort of poor risk, relapsed/refractory lymphoma pts treated consecutively with allogeneic stem cell transplantation over a > 10-year period at our institution with the following observations:
These data clearly demonstrate that a sizable subset of pts with either HL or aggressive NHL (MCL, DLBCL, PTCL) can enjoy long disease-free survival following allo SCT (beyond the expectation of standard salvage chemotherapy).
Relapse following autologous SCT should not preclude consideration of allo SCT as second salvage.
New strategies to improve outcome include our ongoing TH2 study which led to reduced GVH incidence and promising outcome.
Our ongoing study looking at expanded donor derived T-cells is showing promising results in reducing GVH incidence and improving outcome, including in aggressive NHL, and will be presented on a separate presentation.
Mato:Celgene: Speakers Bureau; Millennium: Speakers Bureau; Seattle Genetics: Speakers Bureau; Genentech: Speakers Bureau. Goldberg:Eisai: Speakers Bureau. Feldman:Allos: Speakers Bureau; celgene: Speakers Bureau; Seattle Genetics: Speakers Bureau; Merck: Speakers Bureau. Goy:Milennium: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; J & J: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal