Abstract
The development of a risk-stratification model that relies on molecular cytogenetic markers to assess disease aggressiveness and provide therapeutic guidance would be beneficial for the management of patients with multiple myeloma (MM). High-risk cytogenetic abnormalities, including deletion 17p (del17p), are known to confer a poor prognosis. Intensified consolidation with high-dose therapy (HDT) and autologous stem cell transplantation (ASCT) following induction therapy has been proposed as the preferred initial treatment for high-risk MM, such as del17p. With this background, we report the impact of del17p on the timing and outcome of transplant in patients with MM.
We performed a retrospective review of 226 patients with MM who underwent HDT/ASCT at the University of California, San Diego Moores Cancer Center between 1/2000 and 7/2011. Conventional cytogenetic analyses were utilized for all patients either at diagnosis or at relapse, but prior to ASCT. Fluorescence in situ hybridization was also assessed when available. The primary objective was to evaluate the impact of del17p, a known high-risk chromosomal abnormality, on the overall survival (OS) and progression-free survival (PFS) after ASCT, compared with patients without chromosomal abnormalities. A secondary objective was to assess the OS and PFS for patients with del17p who received ASCT within a year of diagnosis, compared with those who underwent ASCT more than 12 months from diagnosis.
In 226 patients with conventional cytogenetic data prior to ASCT, chromosomal abnormalities were noted in 82 (36%) patients. Of these, 21 (25%) patients harbored a del17p abnormality. Table 1 shows the patient characteristics and ASCT outcomes.
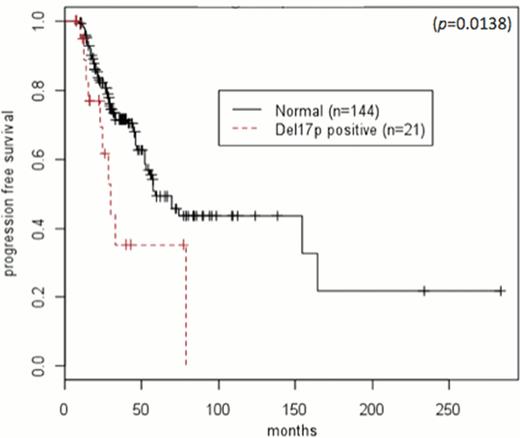
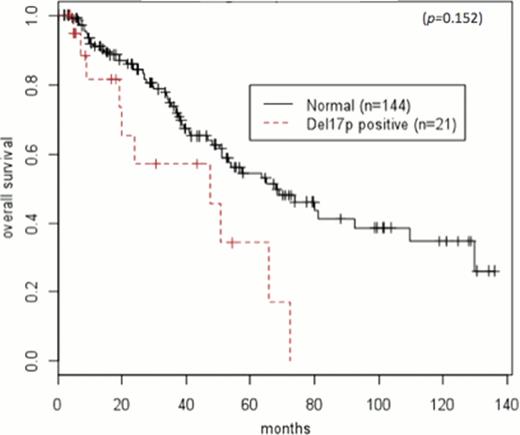
Prior to undergoing ASCT, 4 (19%)patients with del17p achieved a complete remission (CR) or stringent complete remission (sCR), and 6 (29%) achieved a CR or sCR after ASCT. In patients without chromosomal abnormalities, 19 (13%) achieved CR or sCR prior to ASCT, and 45 (31%) achieved it after ASCT. Median follow-up of surviving patients was 47 months (range 5–283). Median PFS after ASCT in patients with del17p and normal cytogenetics were 8.3 months (95%CI: 4.6-N/A) and 33 months (95%CI: 21.7–61.8), respectively (HR 2.15, 95%CI: 1.19–3.89; p=0.011) (Figure 1). Median OS after ASCT in patients with del17p and normal cytogenetics were 47.5 months (95%CI: 19.9-N/A) and 68 months (95%CI 51.1-N/A), respectively (HR: 2.27, 95%CI 1.15–4.48; p=0.018) (Figure 2). Median PFS in patients with del17p who received an ASCT after 12 months and within 12 months from diagnosis was 6.13 months (95%CI: 2.7-NA) and 22.6 months (95%CI: 5.4-NA), respectively (HR=1.22, 95%CI 0.40–3.82; p=0.71). Median OS in del17p patients who received an ASCT after 12 months and within 12 months from diagnosis was 65.6 months (95%CI: 19.3-NA) and 47.5 months (95%CI: 19.9-NA), respectively (HR=0.97, 95%CI 0.26–3.62; p=0.96).
In this single center study with long follow up, we demonstrate that a del17p abnormality in MM confers a shorter PFS and OS after ASCT. Furthermore, the use of HDT/ASCT as part of the upfront treatment plan within 12 months of diagnosis does not significantly affect the outcome in patients with a del17p abnormality. Further studies are required to better define a risk-stratified treatment plan for this subset of high-risk multiple myeloma.
Patient Characteristics
| . | Normal (n=144) . | Deletion 17p (n=21) . |
|---|---|---|
| Median Age at ASCT | 59 | 57 |
| Age 365 at ASCT | 39 | 6 |
| Race | White = 96 (67%) | White = 12 (57%) |
| Black = 11 (8%) | Black = 3 (14%) | |
| Hispanic = 28 (19%) | Hispanic = 5 (24%) | |
| Other = 9 (6%) | Other = 1 (5%) | |
| Gender | Female = 52 (36%) | Female = 7 (33%) |
| Male = 92 (64%) | Male = 14 (67%) | |
| CR Pre-ASCT | sCR/CR = 19 (13%) | sCR/CR = 4 (19%) |
| Response to ASCT | sCR/CR = 45 (31%) | sCR/CR = 6 (29%) |
| 3PR = 68 (48%) | 3PR = 5 (24%) | |
| SD = 26 (18%) | SD = 6 (28%) | |
| PD = 3 (2%) | PD = 3 (14%) | |
| Unknown = 2 (1%) | Unknown = 1 (5%) | |
| PFS | Median = 33 months | Median = 8.3 months |
| OS | Median = 68 months | Median = 47.5 months |
| . | Normal (n=144) . | Deletion 17p (n=21) . |
|---|---|---|
| Median Age at ASCT | 59 | 57 |
| Age 365 at ASCT | 39 | 6 |
| Race | White = 96 (67%) | White = 12 (57%) |
| Black = 11 (8%) | Black = 3 (14%) | |
| Hispanic = 28 (19%) | Hispanic = 5 (24%) | |
| Other = 9 (6%) | Other = 1 (5%) | |
| Gender | Female = 52 (36%) | Female = 7 (33%) |
| Male = 92 (64%) | Male = 14 (67%) | |
| CR Pre-ASCT | sCR/CR = 19 (13%) | sCR/CR = 4 (19%) |
| Response to ASCT | sCR/CR = 45 (31%) | sCR/CR = 6 (29%) |
| 3PR = 68 (48%) | 3PR = 5 (24%) | |
| SD = 26 (18%) | SD = 6 (28%) | |
| PD = 3 (2%) | PD = 3 (14%) | |
| Unknown = 2 (1%) | Unknown = 1 (5%) | |
| PFS | Median = 33 months | Median = 8.3 months |
| OS | Median = 68 months | Median = 47.5 months |
ASCT: Autologous Stem Cell Transplant; sCR: Stringent Complete Remission; CR: Complete Remission; PR: Partial Remission; SD: Stable Disease; PD: Progressive Disease; PFS: Progression Free Survival; OS: Overall Survival
No relevant conflicts of interest to declare.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal