Abstract
Abstract 3075
Allogeneic stem cell transplantation (allo-SCT) is a potential curative therapy for patients with relapsed or refractory acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). However, the risk for disease recurrence following transplant remains high. The ability to identify patients likely to relapse may allow for preemptive interventions in high-risk patients. The goal of hematopoietic transplantation is to eradicate the recipient myeloid leukemia cells and restore hematopoiesis and immunity with donor cells. Donor lymphoid cells mediate an important graft-vs.-leukemia effect. Post transplant peripheral blood (PB) chimerism analysis represents one potential tool for predicting disease recurrence, although the relationship between mixed chimerism and disease relapse is not well defined.
We conducted a retrospective review of patients with AML/MDS who underwent allo-SCT with fludarabine/busulfan based conditioning regimens at The University of Texas MD Anderson Cancer Center between 2001 and 2011. PB chimerism was assessed between post-transplant days +90–120 using a multiplex PCR-based microsatellite polymorphism assay. Cox's proportional hazards regression was used on univariate and multivariate (MV) analysis to evaluate the impact of chimerism of T-lymphocytes and myeloid cells in PB, as well as patient-, disease-, and transplant-related variables on the rate of disease progression and progression-free survival (PFS). Survival and PFS were assessed in landmark analysis starting on day +120. The cut off levels for assessment of chimerism were based on the respective quartiles of distribution of T-lymphocytes and myeloid cells in the study population.
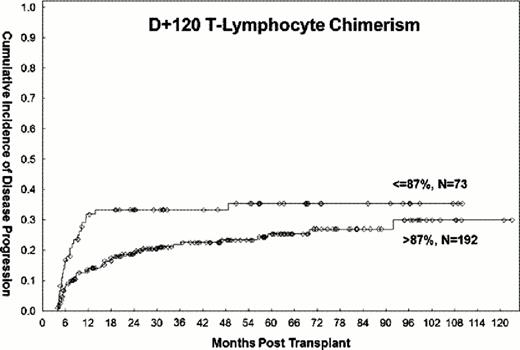
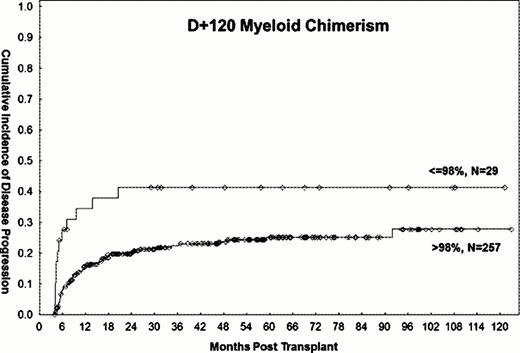
483 patients who underwent allo-SCT for AML/MDS with fludarabine/busulfan based conditioning regimens between 2001–2011 were analyzed. Within this cohort, 378 patients were alive and without evidence of disease progression on day +120 and were eligible for study evaluation. Patients with disease progression or death within 3 weeks of chimerism assessment were excluded from analyses assessing the impact of chimerism on outcomes. 158 patients were in CR1, 66 in CR2, and 154 had active disease at the time of transplantation. PB T-lymphocyte and myeloid donor cell chimerism data between days +90–120 were available for 265 (70%) and 286 (76%) patients, respectively. The median follow-up time among surviving patients was 54 months (range, 5–126). Progression-free survival from day +120 was 56% (95% CI 39–52) at 3 years, and 46% (95% CI 39–52) overall. On univariate analysis, mixed T-lymphocyte chimerism of ≤87% (HR=1.8, P 0.03, Figure 1) and myeloid chimerism ≤98% (HR=2.4, P 0.005, Figure 2) were significantly associated with a higher rate of 3-year disease progression. These cut-off points were based on the 25th percentile of the respective distributions of T-lymphocyte and myeloid chimerism in the study population. Additional adverse factors included poor-risk cytogenetics (HR=1.5, P 0.04) and disease status other than first or second remission at the time of transplant (HR=2, P 0.002). All factors remained significant on MV analysis, with the exception of myeloid chimerism, which became only marginally significant (HR=1.96, P 0.06). Mixed T-lymphocyte (HR=1.5, P 0.05) and myeloid (HR=1.9, P 0.02) chimerism were also associated with significantly lower 3-year PFS on univariate analysis, but were no longer significant (HR=1.5, 95% CI 0.95–2.3 and HR=1.6, 95%, CI 0.9–2.8, respectively) after adjustment for disease status at transplant. Disease status other than first or second remission at transplant was the only significant predictor of 3-year PFS on MV analysis (HR=1.6, P=0.03). Stem cell source (PB vs. BM), donor type (match-related donor vs. other), age (>50 vs. ≤50 years), and diagnosis (AML vs. MDS) did not impact the rate of disease progression or disease free survival.
Mixed T-lymphocyte and myeloid chimerism assessed on day +120 post SCT are associated with the rate of disease progression independently of disease status at transplant. The use of chimerism assessments may be useful in selecting patients at high-risk for relapse for preemptive therapeutic approaches.
Champlin:Otsuka: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal