Abstract
Abstract 3023
The segment attached to the freezing bag is considered an important source of cells for testing the quality of a cryopreserved CBU prior to release for transplantation. CB banks test cells from the segment for CD34+ cell count, viability and colony forming units (CFU) as surrogates of potency of the frozen products. The NCBP reported results of 384 segments of CBU processed with the AutoExpress (AXP) system (Albano et al, ASH 2011). Average segment CD34+ viability was 96% (SD:+/− 3.3%) and correlation with the CBU pre-cryopreservation viable CD34+ counts (vCD34; R2:0.9) and CFU (R2:0.6) were excellent. Segment vCD34+ and CFU also correlated highly with each other (R2:0.69). As of 08/2012, 1056 segments from NCBP AXP CBU have been evaluated with average CD34+ cell viability of 96% (SD:+/− 3.1). However, how the segment results compare to those obtained from the CBU at the transplant center is not established.
To evaluate whether the segment could predict the post-thaw CBU vCD34+ counts, viability and CFU at MSKCC, we compared the post-thaw results of 37 NCBP CBU, AXP-processed and stored in BioArchive freezers, shipped, and thawed at MSKCC, with the information from their respective segments tested at NCBP prior to CBU release and the pre-cryopreservation data. Segment CD34+ counts and viability were evaluated by flow cytometry and 7-AAD exclusion using a single platform and the ISHAGE gating strategy. Segment CFU were evaluated using the NCBP CFU high resolution digital imaging technology (Albano et al, ASH 2008). The segment viable cells/ul were used to estimate the total vCD34 and CFU for the respective CBU. At MSKCC, CBU underwent thaw and albumin reconstitution with 8-fold dilution (10% Dextran 40; 25% albumin) as previously reported (Barker et al, BBMT 2009;15(12):1596–602). Duplicate samples were evaluated by flow cytometry within two hours. Four color flow cytometry using a dual platform was performed to measure CD45+/CD34+/CD3+ cell counts; CD34+ cell viability was assessed using a modified ISHAGE strategy (Scaradavou et al, BBMT 2010;16(4):560–8). CFU assays were performed using 1×105cells plated in duplicate and growth was evaluated at 14 days. All CBU were part of double unit grafts.
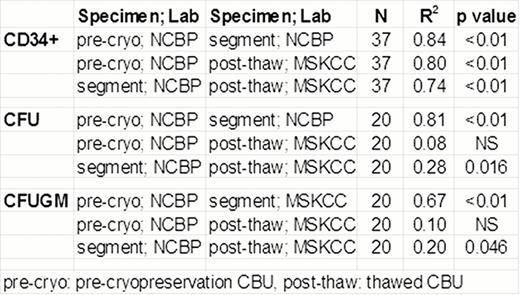
Consistent with prior NCBP data, segment vCD34+ cell counts correlated well with segment CFU (R2:0.89, p< 0.01; N=21). Additionally, high correlation of vCD34+ cell counts and CFU were seen between the pre-cryopreservation CBU and the segment (p<0.01, Table).
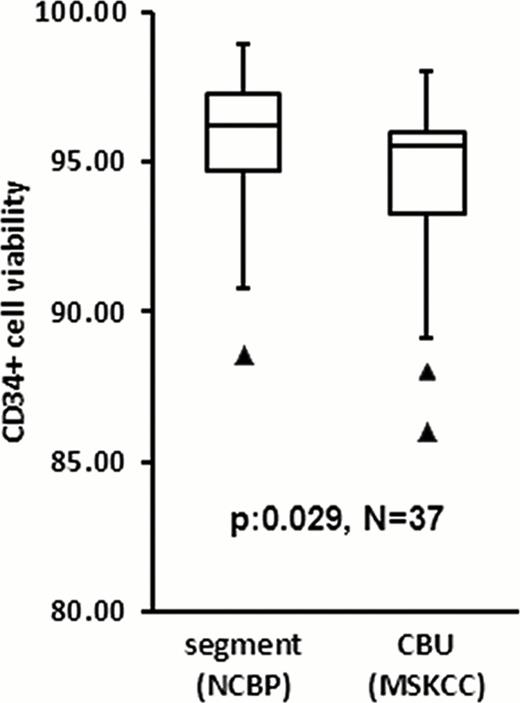
Importantly, despite the differences in testing laboratories and gating strategies, the number of vCD34+ cells in the CBU post-thaw correlated with the pre-cryopreservation vCD34+ counts, as well as those from the segment (Table). Average decrease in post-thaw CBU CD34+ cell viability compared to that of the segment was 1.4% (SD:+/− 3.7%, N: 37). Although statistically significant (p: 0.029), this difference was not clinically relevant: the range of change was -10% to +4.8% and the lowest CBU CD34+ viability was 86% (Figure). Post-thaw CBU total CFU and CFUGM did not correlate with pre-cryopreservation values (Table), probably reflecting high inter-laboratory assay variability. Segment CFU and CFUGM correlation with the post-thaw values was significant although the R2was weak. The ratio of post-thaw to segment vCD34 cells (median: 1.3; SD:+/− 0.47) indicated that the segment calculation may underestimate CBU cell content. The median ratio of post-thaw to segment colony-forming cells was 0.54 for total CFU (SD:+/− 0.63) and 0.5 for CFUGM (SD:+/− 0.96). The lower CFU than vCD34 ratio may be explained, in part, by the fact that 7-AAD detects dead cells but not apoptotic; apoptotic cells are counted as alive by flow cytometry but do not have functionality and do not generate CFU in culture.
Our results indicate that testing of CBU segments can measure accurately the potency of the frozen CB products. Moreover, the results demonstrate that CBU quality can be maintained following albumin-dextran reconstitution. These findings reflect the cryopreservation procedures, freezing bags and reconstitution method described; whether they can be applied to other CB banks or transplant center laboratories requires further investigation.
Correlation of MSKCC post-thaw CBU results with NCBP pre-cryopreservation CBU and segment evaluation
Comparison of segment and post-thaw CBU CD34+ cell viability
Comparison of segment and post-thaw CBU CD34+ cell viability
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal