Abstract
Abstract 2975
Meta-analyses (MAs) with few participants (i.e. small number of primary studies) are at risk of producing random errors and consequently overestimating treatment effects. With insufficient information the risk of obtaining a false positive result (type I error) increases, which may lead to false conclusions. Trial sequential analysis (TSA) has been proposed as a method to ascertain whether results of MAs are conclusive (true vs. false positive, true vs. false negative). It adjusts for the risk of random error by constructing monitoring boundaries under the sample size necessary to conclude significant treatment effect. In TSA, the information size is calculated based on a pre-specified event rate in the control group, a minimum intervention effect (risk ratio reduction), and a desired maximum risk of type I error α and type II error β. Here we apply TSA on MAs of randomized controlled trials of maintenance therapies in the management of multiple myeloma.
A comprehensive literature search of MEDLINE (PubMed), the Cochrane Central Register of Controlled Trials (CENTRAL), and meetings abstracts from American Society of Hematology, American Society of Clinical Oncology, European Society for Medical Oncology and European Hematology Association was undertaken to identify all phase III randomized controlled trials (RCTs) of maintenance therapy published until July 2012. We extracted data on overall survival and progression-free survival comparing treatments that could be pooled in random effects meta-analysis. We performed TSA for the apriori diversity-adjusted information size (APDIS) under risk ratio reduction of 20% and 25%. The information size was adjusted for between-study trial diversity, which is defined as the total relative variance expansion changing from a fixed effect into a random effects meta-analysis. We used two-sided α = 5% and 1 – β = 80% power. All analyses were done in Stata 11.2 using metacumbounds command.
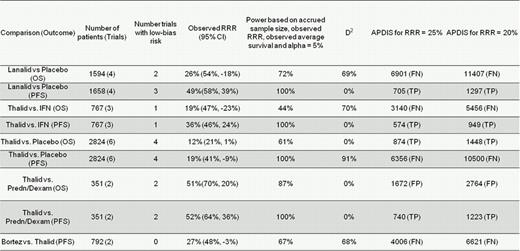
Nine separate meta-analyses (18 randomized controlled trials) met the inclusion criteria (Table 1). The median number of patients was 1193 (range 351–2824) and median diversity 0% (range 0%-91%). Under both risk ratio reductions of 20% and 25%, 4/9 MAs were false negative and 1/9 false positive. The observed power based on the accrued sample size and observed risk ratio reduction was greater than 80% in 5/9 MAs.
TSA detected one false positive MA of two trials comparing thalidomide with prednisone/dexamethosone for the outcome of overall survival. Future MAs need to consistently undertake TSA to avoid misleading conclusions.
Nine meta-analyses of maintenance therapies for multiple myeloma.
APDIS, the apriori diversity-adjusted information size needed to detect a realistic treatment effect given pre-specified risk ratio reduction. Negative risk ratio reduction corresponds to increased risk. TP = True Positive, FP = false positive, TN = true negative, FN = false negative. NP means that TSA is not possible due to a small calculated information size. D2 = Diversity.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal