Abstract
Abstract 2955
Deacetylase (DAC) inhibitors show promise as anti-neoplastic agents, the approved drugs are weak inhibitors of class I and II DACs or potent inhibitors of class I DAC only, and have suboptimal activity or unacceptable toxicities. AR-42 is a class I/II DAC-I designed at OSU that demonstrates a 20,000-fold improvement in DAC inhibitory potency relative to the parent molecule (IC50=16 nM) with greater antiproliferative effects than Vorinostat in vitro and in vivo (Kulp et al, Clin Cancer Res, 2006 and Lucas et al, PLoS One, 2010).
OSU 09102 (NCI 9119) is a first-in-man single agent, cohorts-of-3 phase I dose escalation study in adult patients with relapsed CLL, lymphoma (NHL), or multiple myeloma (MM) with normal kidney and liver function. Patients received AR-42 orally M-W-F in cycles of 28 days (3 weeks of 3-times-per-week dosing followed by a 7-day break). Moderate cell count suppression was allowed with an absolute neutrophil cutoff of 1000/μL, platelets 3 50,000/μL and hemoglobin 3 10 g/dL. In the first stage of dose escalation, each dose level increased by 100% until the first grade 2, drug-related toxicity was observed. Subsequent dose increases will be approximately 33% increase with accrual in cohorts of 3 patients. For pharmacokinetic analysis, plasma was obtained at 0 (pre-dose), 0.25, 0.5, 1, 1.5, 2, 4, 8, 10, 24, and 48 hours after dosing on day 1 and day 19 (only up to 24 h), and then kept at –80°C until analysis.
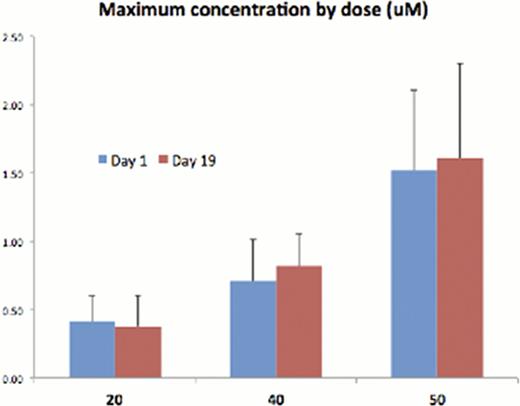
We enrolled 3 patients at 20 mg (MM, MM, NHL), 3 patients at 40 mg (MM) with a transition to a slower dose escalation due to a grade 2 thrombocytopenia. Three more patients were enrolled at 40 mg (MM, MM, T-cell NHL), then 7 patients at 50 mg (MM × 4, follicular × 1, T-cell NHL × 2). One myeloma patient was enrolled at 70 mg. In the 40 mg cohort, related toxicities include 2 grade 3 and 2 grade 2 thrombocytopenia, 1 grade 3 neutropenia, 1 grade 2 vomiting, and 2 grade 1 QTc prolongation. In the 50 mg cohort 1 grade 4 and 3 grade 3 thrombocytopenia, 2 grade 3 neutropenia, 4 grade 2 fatigue, 2 grade 2 muscle spasm, 1 grade 2 blurred vision/dizziness, 3 grade 1 QTc prolongation, and 3 grade 1 nausea. Accrual was temporarily halted for a safety analysis Mar-2012 focused on the 50 mg cohort toxicities – one grade 4 thrombocytopenia considered a DLT, one patient found dead on cycle 2 day 10 without prior evidence of QTc prolongation, and one patient with reproducible dizziness and blurry vision. AR-42 was detected 15 mins after dose in 12 of 17 patients, suggesting rapid absorption. The time to reach the peak concentration in plasma (Tmax) varied from 1.5 hours to 4 hours. The Cmax (see chart) and AUC of AR-42 was not increased proportionally with doses, suggesting that the PK of AR-42 is not linear in the 20–50 mg range.
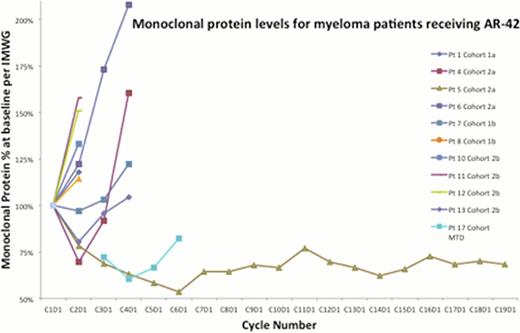
The Cmax achieved at the 40 mg and 50 mg dose levels is adequate for HDAC inhibition in vitro and minor clinical responses were observed in myeloma and T-cell lymphoma as a single agent in the 40 mg cohort (see monoclonal proteins chart), hence 40 mg TIW 3-weeks-on and 1-week-off was declared the MTD. Complete pharmacokinetic, toxicity, and results from brief fatigue inventory will be presented at the meeting. AR-42 does not have the severe fatigue and gastrointestinal side effects of other broad DAC inhibitors and may be suitable for combination phase Ib trials in T-cell lymphoma and myeloma.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal