Abstract
Abstract 2922
Osteolytic bone disease (OBD) in multiple myeloma (MM) is caused by a combination of osteoclast hyperactivation and osteoblast inhibition. Several studies have shown that the Wnt-inhibitor DKK1 is one of the factors involved in osteoblast inhibition (Tian, NEJM, 2003), while the role of other Wnt-pathway inhibitors, such as secreted frizzled related protein-2 (SFRP2), SFRP3, sclerostin and Wnt inhibitory factor 1 (WIF1) are less studied. In addition stromal derived factor 1 (SDF-1/CXCL12) that interacts with CXCR4 in bone-marrow (BM) is a potential factor in OBD, although conflicting data has been reported. SDF-1has in mesenchymal stromal cells (MSC) been found downregulated in MM patients compared to healthy volunteers (HV) and also downregulated in MM plasma cells vs. normal plasma cells, while SDF-1 serum levels has been correlated to the extent of OBD in MM.
Until now, gene expression studies in MM have been performed on isolated MM plasma cells or bone marrow aspirates, which are not completely representative of the cell composition in the BM micro-environment. We used a novel strategy whereby BM biopsies are snap-frozen and subsequently gene expression is analyzed. Using this strategy we have examined the gene-expression of Wnt inhibitors and SDF1. In addition, we have evaluated the protein levels of DKK1 and SDF-1 in matched BM plasma samples.
An additional BM core biopsy obtained during the diagnostic procedure of MM patients was snap-frozen. Biopsies were cut, homogenized and RNA was purified and analyzed by qRT-PCR using low density arrays (Applied Biosystems). The relative quantitative gene expression was calculated using 3 internal reference genes (ABL, GAPDH and GUS). OBD was evaluated using standard radiographs. All patients were untreated and did not receive medicine that could influence bone remodeling. We examined 10 healthy volunteers (HV), 35 monoclonal gammopathy of unknown significance (MGUS) and 65 untreated MM patients, which according to radiographic findings were divided into NO/LOW and advanced OBD, i.e. OBD in ≥2 regions.
ELISA was performed on a total of 31 matched BM plasma samples of HV, MGUS and MM obtained at the same time point as the biopsies. In addition, extra samples without gene data (N=52) were analyzed. Commercial kits for DKK-1 (RnD, Quantikine) and SDF-1 (RnD, Quantikine) were run in duplicates according to manufacturer's instructions.
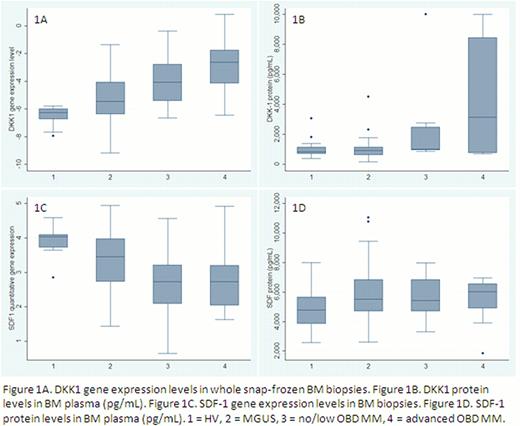
Gene expression of DKK1 and SFRP3 were significantly (p<0.05) associated with OBD with increasing gene-expression with increasing OBD, while SDF-1 were significantly associated with decreasing gene-expression with increasing OBD. Gene expression of SFRP2, sclerostin and WIF1 did not associate with the extent of OBD. When comparing HV, MGUS and MM independent of OBD, sclerostin showed a tendency to be higher expressed in MM vs. MGUS and HV (p=0.08).
A significant correlation between gene expression levels and protein expression levels was found for DKK-1 (Spearman's rho= 0.57, p=0.0006,) while the correlation of gene and protein expression levels of SDF-1 did not reach significance (Spearman's rho =0.31, p=0.074).
The protein level of DKK1 in BM plasma was upregulated in MM and associated significantly with the OBD level (p<0.05). The protein level of SDF1 did not correlate to the extent of OBD.
Our gene expression study of MM with minimal manipulation of the samples showed that the Wnt-inhibitors DKK1 and SFRP3 were significantly associated to the extent of OBD, while SDF1 gene expression inversely correlated with the extent of OBD. The report of simultaneous measurement of not only DKK1 but also SFRP3 and other Wnt-inhibitors is novel. The use of whole snap-frozen BM biopsies is a new method to evaluate gene expression in MM that makes it possible to include the tumor micro-environment and investigate patients independent of degree of MM plasma cell infiltration, and we were able to reproduce the DKK1 findings by others. The gene expression results were complemented by protein data for DKK1 and SDF-1. Our study shows for the first time the difference between SDF1 gene and protein levels in matched MM patient samples, and the difference measured incites us to further investigate the post-translational regulation of SDF-1, and the possibility of SDF1 protein being less bound to CXCR4 in MM.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal