Abstract
Abstract 2843
The hypomethylating agents (HMAs) 5-azacitidine (AZA) and decitabine (DAC) provided significant overall response rates (40–60%) in myelodysplastic syndrome (MDS), and improved the outcome of higher risk MDS. However, phase III trials comparing AZA or DAC to conventional treatment including best supportive care have shown discrepant results. The aim of this study is to compare the efficacy and safety between AZA and DAC in patients with MDS.
We evaluated 203 patients in lower risk with significant cytopenia and higher risk MDS who received AZA and 97 patients who received DAC in Korea between January 2004 and December 2011. AZA 75mg/m2/day was given subcutaneously for 7 days every 4 weeks. DAC 20mg/m2/day was given intravenously over one hour for 5 days every 4 weeks. We compared overall response rate (complete responses, partial responses, marrow complete responses, and hematologic improvements), overall survival (OS) and adverse outcomes with the use of propensity-score matching in the overall cohort according to HMAs.
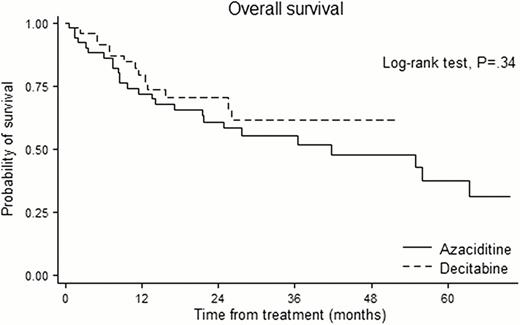
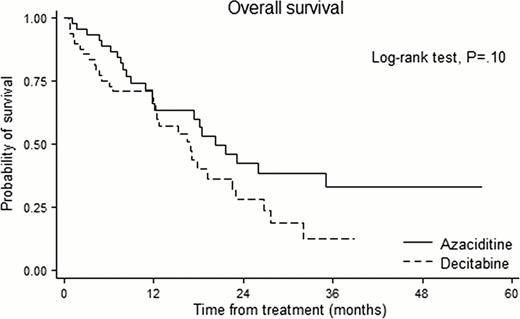
Among 300 patients, propensity matching for the entire cohort created 97 matched pairs of patients. The International Prognostic Scoring System risk category was Intermediate-2/High in 40.2%. A median of 5 courses (range 1–24) were delivered in AZA and 5 courses (range 1–14) in DAC. In the overall matched cohort, there was no significant difference between AZA and DAC in overall response rate (44.2% vs. 52.1%, P=.28), OS (28 vs. 23 months; hazard ratio for AZA, 1.14; 95% confidence interval [CI], 0.75 to 1.72, P=.54) with a median follow-up duration of 29.6 months. Among the patient under 65, no significant differences were noted for OS between AZA and DAC group. Among the patient over 65, however, the patients who received DAC showed higher risk of death than those who received AZA with borderline significance (hazard ratio for AZA, 1.58; 95% CI 0.91 to 2.73, P=.10). The cumulative hazard of transformation to acute myeloid leukemia (AML) was 16.3% in AZA and 21.9% in DAC at one year, and 32.2% in AZA and 55.3% in DAC at two year. The incidence of grade 3 & 4 neutropenia was significantly higher in DAC than AZA (P=.026). Among 1151 assessable treatment courses (604 in AZA, 547 in DAC), AZA group have less likely to experience fever episodes requiring intravenous antibiotics than DAC group (8.6 vs. 15.7 episodes per 100 courses; risk ratio, 0.55; P<.001).
In a cohort of patients in lower risk with significant cytopenia and higher risk MDS, AZA and DAC showed comparable efficacy in terms of overall response rate, OS and risk of transformation to AML. However, patients receiving DAC experienced more frequent grade 3 & 4 neutropenia and fever episodes than patient receiving AZA. When both AZA and DAC are available, safety profiles as well as treatment efficacy need to be considered.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal