Abstract
Abstract 2741
The novel agent lenalidomide (LEN) has shown significant single-agent activity in patients with relapsed lymphoma through anti-proliferative and immunomodulatory mechanisms. In combination LEN may synergistically improve the efficacy of rituximab. However, there is limited data on the efficacy and safety of LEN in multi-drug combination regimens for treatment-naïve patients. We designed a phase II single arm trial to evaluate tumor response, toxicity (AE) and survival with a combination of LEN, rituximab, cyclophosphamide (CTX) and dexamethasone (DEX) (LR-CD) in symptomatic untreated patients with indolent B-cell lymphoma. (NCT00784927).
Eligibility required age ≥18, ECOG PS ≤2, confirmed diagnosis of indolent B-cell lymphoma (FL1–2, SLL, MZL or LPL/WM), measurable nodes ≥2cm or IgM ≥400mg/dL (LPL/WM), ANC ≥1400/mm3, platelets ≥100,000/mm3, creatinine ≤2mg/dL, and signed informed consent. Only patients needing therapy were considered eligible. Treatment consisted of IV rituximab 375mg/m2 D1, oral LEN 20mg D1–21, CTX 250mg/m2 D1, 8, 15, DEX 40mg D1, 8, 15, 22 and ASA 325 mg daily in a 28 day cycle. Treatment continued 2 cycles beyond best response up to a maximum of 12 cycles. Toxicity was assessed by NCI CTCAE v3.0.
Thirty-one patients have enrolled at Mayo Clinic with 28 evaluable for toxicity and response (1 deemed ineligible, 1 withdrew before treatment, 1 treatment violation). Four patients continue on study at this time. Patient characteristics: median age 68.5(43–83), 71% male, stage IV 86%, FL 28%, MZL 25%, LPL/WM 39%, and SLL 4%. Median number of cycles given was 6 and 71% completed the study per protocol.
The best overall response is 89% with 32% CR and 57% PR. In subgroup analysis, the response rates for LPL/WM was 91% (all PR) and for FL 88% (CR 63%, PR 25%). The most common grade ≥3 AEs were neutropenia (35.7%), leukopenia (17.8%), anemia (14.3%) and thrombosis (14.3%). The LPL/WM subgroup experienced more grade ≥3 anemia (36%) compared to the group as a whole. Patients (57%) required at least one dose reduction of LEN (n =10), CTX (n = 9) or DEX (n = 7).
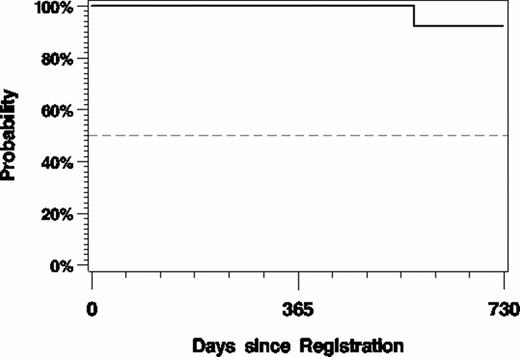
At a median f/u of 14.5 (1.8–36.5) months 86% are progression free (fig. 1) and the OS is 96% (fig. 2). One death unrelated to treatment occurred 7.7 months after completing 12 cycles of treatment.
Lenalidomide can be safely combined with other active agents and in the combination LR-CD produces high response rates in symptomatic patients with indolent B-cell lymphoma. The regimen is well tolerated with manageable toxicities which are similar to that seen with single agent LEN. The encouraging results seen in patients with LPL/WM have led to expansion of this cohort for additional study.
Reeder:Celgene: Research Funding. Off Label Use: lenalidomide use in non-Hodgkin lymphoma. Stewart:Celgene: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal