Abstract
Mantle cell lymphoma (MCL) is an uncommon type of non-Hodgkin's lymphoma (NHL) with poor prognosis that necessitates the development of new treatments. Lenalidomide is a unique immunomodulatory agent with antiproliferative and tumoricidal effects on MCL cells. NHL-003 was a phase II, open-label, multicenter trial for subjects with relapsed aggressive NHL that tested single-agent lenalidomide 25 mg PO days 1–21 every 28 days. The primary endpoint was overall response rate (ORR); secondary endpoints included complete response (CR) rate, duration of response (DOR), survival, and safety. At the time of the initial publication (Witzig et al. Ann Oncol.2011;22:1622–1627), MCL subgroup analysis showed an ORR of 42%; the median DOR had not been reached. The purpose of this report is to provide long-term efficacy and safety results for the MCL subgroup from NHL-003.
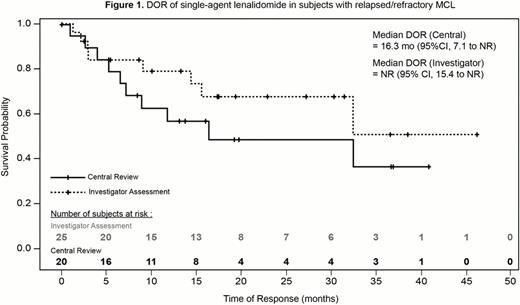
Subjects with MCL (N=57) had a median age of 68 y (range, 33–82), were predominantly male (77%) with good ECOG performance status (89% PS 0–1) and advanced-stage disease (88% stage III/IV). Subjects had received a median of 3 (range, 1–13) prior systemic therapies. According to the current central review at a median follow-up of 12.4 mo, subjects achieved an ORR of 35% (CR/CRu 12%) following lenalidomide, including a median DOR of 16.3 mo (Table 1). The ORR to single-agent lenalidomide was 44% (CR/CRu 21%) by independent assessment, with a median DOR not yet reached. Median PFS was 8.8 mo by central review and 5.7 mo according to investigators. Subjects responded quickly, with a median time to first response of 1.9 mo (central and investigator). Median DOR for subjects in CR and overall survival for all subjects have not yet been reached. The most common grade 3/4 adverse events (AEs) were neutropenia (46%), thrombocytopenia (30%), fatigue (9%), and diarrhea (5%). Other AEs included one subject with grade 1 to 2 tumor flare reaction, one subject with grade 3 deep vein thrombosis, and two subjects with second primary malignancies suspected of being related to treatment (one grade 3 squamous cell carcinoma of the skin that resolved and one grade 4 AML in a heavily pretreated individual with 5 prior cancer therapies).
This subset analysis from a phase II study further confirms the efficacy of lenalidomide in subjects with relapsed MCL. Responders have a long DOR with manageable side effects. These results support continued investigation of lenalidomide alone or in combination for the treatment of MCL.
Efficacy of single-agent lenalidomide in subjects with relapsed/refractory MCL
| Efficacy Parameter (N=57) . | By Central Review . | By Investigator Review . |
|---|---|---|
| ORR, n (%) | 20 (35) | 25 (44) |
| CR/CRu, n (%) | 7 (12) | 12 (21) |
| PR, n (%) | 13 (23) | 13 (23) |
| Median DOR, mo | 16.34 (95% CI, 7.1 to NR) | NR (95% CI, 15.4 to NR) |
| Median time to first response, mo (range) | 1.9 (1.6–24.2) | 1.9 (1.6–15.2) |
| Median PFS, mo | 8.8 (95% CI, 5.5–23.0) | 5.7 (95% CI, 2.7–10.7) |
| Efficacy Parameter (N=57) . | By Central Review . | By Investigator Review . |
|---|---|---|
| ORR, n (%) | 20 (35) | 25 (44) |
| CR/CRu, n (%) | 7 (12) | 12 (21) |
| PR, n (%) | 13 (23) | 13 (23) |
| Median DOR, mo | 16.34 (95% CI, 7.1 to NR) | NR (95% CI, 15.4 to NR) |
| Median time to first response, mo (range) | 1.9 (1.6–24.2) | 1.9 (1.6–15.2) |
| Median PFS, mo | 8.8 (95% CI, 5.5–23.0) | 5.7 (95% CI, 2.7–10.7) |
CR(u) = complete response (unconfirmed); DOR = duration of response;
NR = not reached; PFS = progression-free survival; PR = partial response
Zinzani:Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Millenium: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Mundipharma: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees; Spectrum: Membership on an entity's Board of Directors or advisory committees. Off Label Use: This is a phase 2 clinical study of safety and efficacy for lenalidomide in patients with MCL. Czuczman:Celgene: Consultancy, Consultant Celgene Advisory Board Other. Reeder:Celgene: Mayo Clinic receives funding from Celgene to support clinical trials Other, Research Funding. Haioun:Celgene: Celgene Advisory Board Consultant Other, Consultancy. Tilly:Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding. Pietronigro:Celgene: Employment. Ervin-Haynes:Celgene: Employment. Li:Celgene: Employment. Witzig:Celgene: Research Funding.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal