Abstract
Abstract 2736
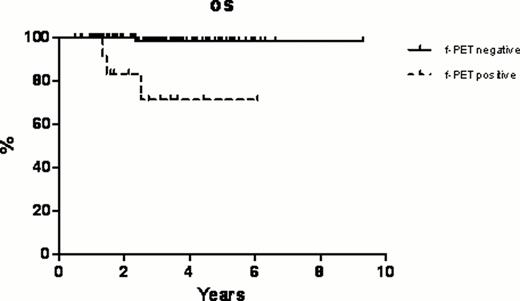
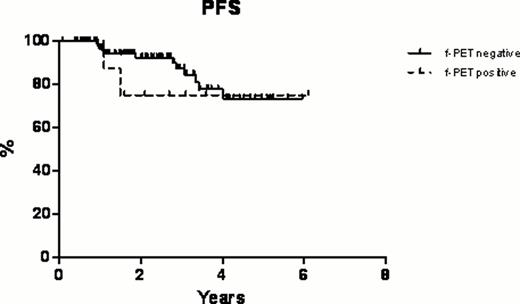
In this retrospective single-center study we aimed at evaluating the efficacy and safety of fludarabine, mitoxantrone and rituximab (FMR) regimen as first line therapy in untreated patients with follicular non-Hodgkin lymphoma (NHL) and indolent non-follicular NHL considering also the role of positron emission tomography (PET) after this chemo-immunotherapy induction as predictor of survival. Between January 2000 and May 2011, 285 patients with stage II-IV untreated indolent follicular (excluding grade IIIb) NHL (n=142) and indolent non-follicular (including marginal zone lymphoma, MZL [n=111] and small lymphocytic lymphoma, SLL [n=31]) NHL (n=143) were diagnosed and treated at our institution in the outpatient clinic. Median age was 63 years (range, 25–83 years) and the median time from diagnosis to study entry was 3 months (range, 1–5 months). 20 patients had stage II, 75 patients had stage III, and 190 had stage IV disease (155 patients had bone marrow involvement). Standard fludarabine (25 mg/m2 iv on days 2, 3 and 4), mitoxantrone (10 mg/m2 iv on day 2) and rituximab (375 mg/m2 iv on day 1) were given every 28 days for six cycles. Globally, after FMR regimen, the overall response rate (ORR) was 83.2%, including a 71.6% complete remission (CR) rate (204 patients) and a 11.6% partial remission (PR) rate (33 patients). According to the histology, in the follicular subset, the ORR was 81.1% with a CR rate of 69.2% while in the indolent non-follicular subset the ORR was 85.2% with a CR rate of 73.9%. In particular, in the indolent non-follicular NHL subgroup the CR rate was 80.2% in MZLs and 51.6% in SLLs, respectively. Toxicities were generally mild and mainly hematologic. Overall 88 (30.8%) patients had grade ≥3 hematologic toxicity, and 26 (9.1%) patients had non-hematologic toxicity with 3 cases of grade ≥3 (1 neurologic toxicity and 2 hepatic toxicity). In terms of secondary malignancies, only 3 (1.0%) hematologic neoplasms were reported (1 myelodisplastic syndrome after 9 months from the end of the treatment and 2 acute lymphoblastic leukemia after 8 and 11 months from the end of the treatment, respectively). Globally with a median follow up of 40 months (range, 12–144 months), at 11 years the overall survival (OS) was 78.8%, the disease-free survival (DFS) was 73.4% (with only 29 relapses), and the progression-free survival (PFS) was 71.9%. Regarding the comparison between the two subsets, follicular vs indolent non-follicular, no statistically significant differences were observed in OS, DFS and PFS curves. Furthermore, a sub-sample of 132 patients (75 follicular NHLs and 57 indolent non-follicular NHLs) had a PET evaluation before the treatment (staging) and 4 to 6 weeks after completion of the sixth cycle of chemo-immunotherapy (restaging, final PET [f-PET]). Post-induction PET-positive patients had a significantly inferior OS at 6 years: 71.4% compared with 98.4% for f-PET-negative patients (p<0.0001, Figure 1a). In terms of PFS at 6 years, there was not a statistically significant difference among f-PET-positive patients and f-PET-negative patients (Figure 1b).
In conclusion, this study suggests and confirms that FMR is a very active, well tolerated (in terms of acute and long-term side effects) chemo-immunotherapy front-line treatment for follicular NHL and indolent non-follicular NHL. PET status at the end of this chemo-immunotherapy induction is quite controversial as a predictor of survival.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal