Abstract
Conflicting results have been published to date concerning the impact of statin use on the prognosis of patients with non-Hodgkin lymphoma (NHL) treated with a rituximab-containing first-line regimen. By impairing protein prenylation and possibly lipid-raft formation, statins have been shown to exert an antitumoral activity both on solid and hematological malignancies in vitro as well as in animal models. However, in vitro experiments have demonstrated an unexpected impairment of anti-CD20 antibody binding due to changes in conformational epitopes of the molecule. As a result, statins were found to decrease anti-CD20 induced complement-dependent cytotoxicity and antibody-dependent cellular cytotoxicity. Several groups worldwide attempted to assess the outcome of patients on statins receiving rituximab for NHL. No detrimental effect was observed across studies for patients with diffuse large B-cell lymphoma treated upfront with R-CHOP or R-CHOP-like regimen but a surprising prolonged event-free survival (EFS) was observed for patients with follicular lymphoma (FL) in one study. However, several caveats of the study precluded definitive conclusions to be drawn such as treatment heterogeneity, the enrollment of patients who did not receive rituximab, the retrospective assessment of statin use through medical charts and the consideration of EFS as the primary endpoint without specific evaluation of overall survival (OS). The impact of statin use on the prognosis of patients with FL therefore requires further investigation.
The randomized, open-label PRIMA study enrolled 1217 patients with de novo FL from 223 centers in 25 countries. Patients achieving at least a partial response following frontline therapy with R-CHOP, R-CVP or R-FCM were randomized between 2-years rituximab maintenance (every 8 weeks) or observation. A significant improvement of progression-free survival (PFS) was observed in the rituximab maintenance arm (HR=0.55, 95% CI=0.44–0.68, p<0.0001). All concomitant treatments at enrollment, including the use of statins, were prospectively collected. 1140 patients with complete medication data available at registration were included in this analysis. Among them, 10.4% (n=119) were on statins. Baseline demographic characteristics between patients on statins and those not on statins were compared with the use of a Chi-square test. Various endpoints such as EFS, PFS, OS, time-to-next lymphoma treatment (TTNLT) and time-to-next chemotherapy (TTNCT) were examined in univariate and multivariate analysis. All time-to-event durations were calculated from the date of randomization (6 months after the start of induction therapy).
As expected, the use of statins was associated to an older age at registration (P<0.001) and, as a consequence, to a higher FLIPI risk score (P<0.001). Male sex was also overrepresented among statins users, although not significant (P=0.07). Unexpectedly, LDH was more frequently above the upper normal limit (P=0.03). No other difference was observed with regards to demographic data according to statin use.
No difference in overall response rate to induction regimen as well as in the quality of the response (CR/Cru versus PR versus others) was noted (P=0.53). No further difference was found at the end of the maintenance period either for patients in the observation or in the rituximab treatment arm. Of note, no imbalance was detected concerning the rate of adverse events between the 2 groups of patients as a whole (grade 1–2 versus 3–4, P=0.18) or with regards to each particular toxicity.
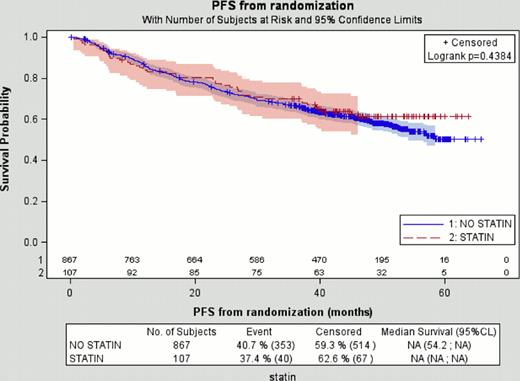
Finally, outcome in terms of EFS, PFS (see figure above), OS, TTNLT and TTNCT were similar regardless of the use of statins (P=0.76, P=0.43, P=0.21, P=0.83 and P=0.31 respectively). Importantly, further adjustment for potential confounding factors in multivariate Cox models such as age, FLIPI score or LDH (which were imbalanced between the 2 groups) did not reveal a potential beneficial effect.
As for other subtypes of NHL, the use of statins in patients with FL in the rituximab era appeared safe and was not associated with an inferior outcome as suggested by in vitro experiments.
No relevant conflicts of interest to declare.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal