Abstract
Abstract 2442
Cyclodextrins (CyDs) are cyclic oligosaccharides that can remove cholesterol from cell membranes and thereby affect receptor function, and are widely used in the pharmaceutical field because of those abilities to improve solubility, dissolution rate and bioavailability of the drugs. A number of studies have demonstrated that methyl-β- cyclodextrin (MβCD) can damage tumor cells and induce cell death. Very recently, Yan et al reported, on the basis of in vitro experiments, that MβCD induces apoptosis of chronic myeloid leukemia (CML) cells and have synergistic anti-leukemic effect combined with imatinib. 2-Hydroxypropyl-β-cyclodextrin (HP-β-CyD) is clinically used as a pharmaceutical excipient, which has been successfully applied to poorly water-soluble drugs. Recently HP-β-CyD has been approved for the treatment of Niemann-Pick type C disease, a rare lysosomal lipid storage disorder.
In the present study, we examined the antiproliferative effect of HP-β-CyD on the in vitro growth of leukemic cell lines and in vivo model using mice transplanted with leukemic cell line. First in vitro proliferation was assessed using the modified MTT assay. The human Ph+ leukemic cell line BV173 and BaF3 cells expressing p190 wild type BCR-ABL (hereafter BaF3/BCR-ABL) were used for evaluation. The growth of BV173 and BaF3/BCR-ABL cells were similarly inhibited by HP-β-CyD in a time- and dose-dependent manner with IC50 value of 4.68 ± 0.98 and 6.01 ± 1.04 mM, respectively. In contrast, IC50 value for hepatocytes was 18.65 ± 4.84 mM, suggesting some therapeutic window between normal cells and CML cells. We next determined if the inhibition of leukemic cell growth by HP-β-CyD was associated with the induction of apoptosis. BaF3/BCR-ABL and BV173 cells were exposed to HP-β-CyD for 12, 24, 48 hours at concentrations of 5, 10, 15 and 25mM. Assessment of apoptosis by 7-AAD/Annexin V double staining revealed that HP-β-CyD induced apoptosis in both cell lines in a time- and dose-dependent manner. The toxicity of HP-β-CyD on normal hematopoietic progenitors was also examined. The susceptibility of normal hematopoieic progenitors was investigated by colony-forming units (CFU-C) assay. When normal progenitors were treated with 5, 15 or 25 mM HP-β-CyD, the percentage of colonies was 92.7 ± 8.6 %, 83.8 ± 23.5 % and 52.4 ± 9.7 % of control, respectively. These results also indicate that HP-β-CyD may not induce bone marrow suppression up to 15mM.
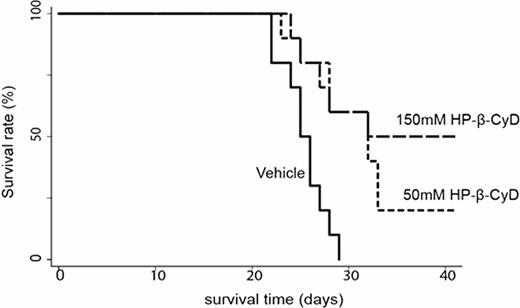
Because in vitro assay showed significant effects against leukemia cell growth, we also investigated the in vivo efficacy of HP-β-CyD. Six-week-old nude mice were injected with 1×106 BaF3/BCR-ABL cells, and were intraperioneally treated with 200 μl of either vehicle, 50mM HP-β-CyD or 150mM HP-β-CyD twice a day (BID) for 20 days from 3days after transplantation. The vehicle-treated mice died of a condition resembling acute leukemia by 29 days after transplantation; HP-β-CyD- treated mice survived more than 40 days, significantly improved the survival (50mM: P=0.003, 150mM: P=0.001, respectively) compared with control mice (Figure 1). These results clearly demonstrate that HP-β-CyD itself has a certain level of anti-leukemic potential. Though further investigations are required to elucidate the mechanisms underlying the antiproliferative function of HP-β-CyD, we should take notice of additional effect when the evaluation of drug efficacy is performed for anti-cancer agents complexed with HP-β-CyD.
Administration of HP-β-CyD prolonged the survival in mice model of BCR-ABL-induced leukemia. Nude mice were injected with 1×106 BaF3 cells expressing p190 BCR-ABL. These mice were treated with vehicle or HP-β-CyD (50 or 150mM) for 20days from 3 days after transplantation.
Administration of HP-β-CyD prolonged the survival in mice model of BCR-ABL-induced leukemia. Nude mice were injected with 1×106 BaF3 cells expressing p190 BCR-ABL. These mice were treated with vehicle or HP-β-CyD (50 or 150mM) for 20days from 3 days after transplantation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal