Abstract
Abstract 2425
Germline heterozygous mutation in the VHL tumor suppressor gene is the underlying genetic defect in VHL disease. Cancer arises when the function of the remaining wildtype allele is lost. VHL patients are prone to develop renal cell carcinoma of the clear cell type (ccRCC) and hemangioblastoma (HB). VHL mutations underlie also the majority of sporadic ccRCC and HB. HB are highly vascularized tumors of the central nervous system. However, it is unclear from which tissue HB arise (reviewed in (1)). Although HB are sometimes described as vascular tumors, inactivation of both VHL alleles has been detected only in the stromal component, suggesting that abnormal angiogenesis is driven by cytokines released from the tumor rather than by VHL inactivation in the endothelium. There is evidence that hematopoietic stem cells or early progenitor cells are involved in the formation of HB. For instance, advanced HB are frequently accompanied by extramedullary erythropoiesis (EME) (2,3).
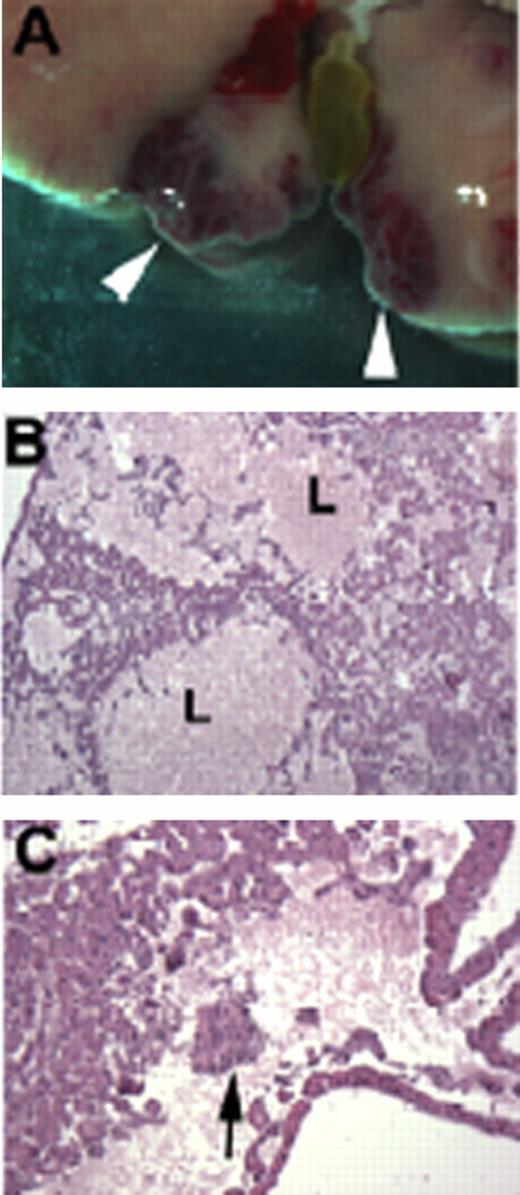
Previously, overgrowth of blood vessels (hemangioma) has been observed in the liver of constitutive heterozygous Vhl knock-out mice, and in a hepatocyte specific Vhl conditional knock-out (albumin-cre) (4). Here, we present a mouse model that may shed light on another aspect of HB, e.g. EME. We used HOXB7-cre for the conditional deletion of VHL in a subset of kidney tubules (HOXB7-Vhl mice). Interestingly, we found that these mice developed not only a kidney phenotype, but also liver lesions. At 2–3 months of age, blood filled bulges developed close to the gall bladder (see Fig. 1A). Superficially, these lesions resembled previously described hemangiomas. However, histology revealed large blood lakunae that were not lined by blood vessels (Fig 1B). Hence, lesions appeared to arise not due to an overgrowth of blood vessels, but due to EME, with massive accumulation of red blood cells surrounding foci of nucleated progenitor cells (Fig. 1C).
Liver lesions in HOXB7-Vhl mice. A: Blood filled bulges form close to the gall bladder (arrowheads). B, C: Histology. Note lakunae of blood (L) without endothelial lining (B), and foci of progenitor cells (arrow in C).
Liver lesions in HOXB7-Vhl mice. A: Blood filled bulges form close to the gall bladder (arrowheads). B, C: Histology. Note lakunae of blood (L) without endothelial lining (B), and foci of progenitor cells (arrow in C).
CFU assays with liver cell suspensions confirmed EME: there was a significant increase in the number of liver BFUs in HOXB7-Vhl mice. HOXB7-Vhl mice had also elevated levels of EPO in the serum, and flow cytometry showed an increase in TER119+CD71+ erythrocyte progenitors in the spleen, but not the bone marrow. Despite EME and elevated EPO levels, HOXB7-VHL mice displayed only rarely polycythemia. In this context, it is noteworthy that VHL patients also present frequently with elevated EPO levels, without developing polycythemia. Interestingly, the majority of HoxB7-VHL mice developed thrombocytopenia, possibly due to the leakiness of the blood vasculature in the liver lesions. However, flow cytometry showed also a decrease in megakaryocytes (CD41+) in both bone marrow and spleen, and in addition to an increase of erythrocyte progenitors, an increase in TER119+CD41+ common megakaryocyte-erythrocyte progenitors (MEP) in the spleen. It is therefore possible that differentiation of the MEP is shifted towards the erythrocyte lineage in the HOXB7-Vhl mouse.
We found no evidence of Vhl deletion in the liver of HOXB7-Vhl mice using LacZ reporter mice. Our mouse model suggests therefore a systemic etiology of HB. One of the best known functions of VHL is the negative regulation of the hypoxia-inducible factor (HIF). Besides EPO, the chemokine receptor CXCR4 is also a known HIF-responsive gene (5) that could be potentially disregulated by Vhl deletion. Furthermore, preliminary data indicate that constitutive heterozygous VHL mice show 50% mortality in transplantation experiments, irrespective of the genotype of transplanted stem cells (e.g. wt or VHL +/−). Hence, in addition to cell autonomous and systemic mechanisms, defects in the bone marrow environment may underlie hematologic presentations of VHL deficiency.
No relevant conflicts of interest to declare.
References:
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal