Abstract
Abstract 2206
Clearance of blood coagulation factor VIII (FVIII) is mediated by several receptors (Saenko E. et al, 1999; Lenting P. et al, 1999 and Sarafanov A. et al, 2001) and one of them was proposed to be low-density lipoprotein receptor (LDLR) (Bovenschen N. et al, 2005). Besides FVIII, the major ligands of LDLR are plasma lipoproteins. The ligand-binding portion of LDLR is represented by a cluster of seven complement-type repeats. Each repeat forms a structurally autonomous domain, which are connected with flexible linkers. Two and five adjacent CRs were shown to form sites for the lipoproteins ApoE and ApoB, respectively, and the site for FVIII has not been determined.
As a part of elucidation of mechanisms of FVIII clearance, our goal was to identify the FVIII-binding region on LDLR.
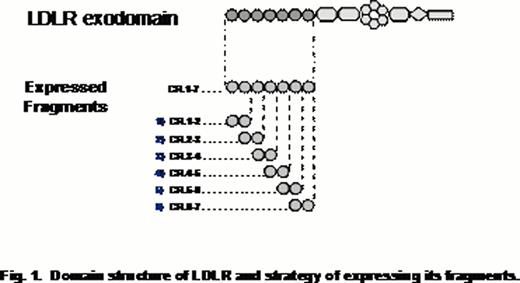
Our experimental strategy was based on generation of recombinant CR-doublets that systematically overlap the CR cluster in LDLR (Fig. 1) and testing their interactions with FVIII. The proteins were expressed in insect cells using a baculovirus system and tested for structural integrity by circular dichroism. The functional properties of the fragments were assessed by their binding with alpha-2-macroglobulin receptor-associated protein (RAP), a universal ligand of a family of receptors that LDLR belongs to. The binding studies were performed using surface plasmon resonance technique. Using this assay, the LDLR fragments were further tested for interaction with different FVIII preparations, such as plasma-derived FVIII and recombinant FVIII: Advate (full-size FVIII) and Xyntha (B-domain deleted FVIII). The specificity of these interactions was tested in a competitive binding assay using an anti-FVIII single-chain variable antibody fragment KM33 known to inhibit binding FVIII to LDLR (Limburg V. et al, 2005). Also, the specificity of the interaction of LDLR fragments and FVIII was tested upon mutating the fragments by targeting conservative tryptophanes, one residue per selected CR. Upon expressing the mutants, their structural adequacy to the respective wild-type forms was confirmed by circular dichroism.
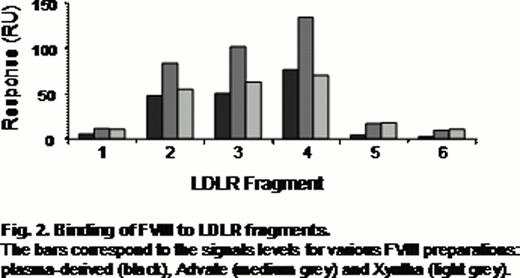
Three overlapping CR doublets were found active for binding with all FVIII preparations (Fig. 2). In presence of increased concentrations of KM33 and upon site-directed mutagenesis of the LDLR fragments, this binding was diminished confirming its specificity.
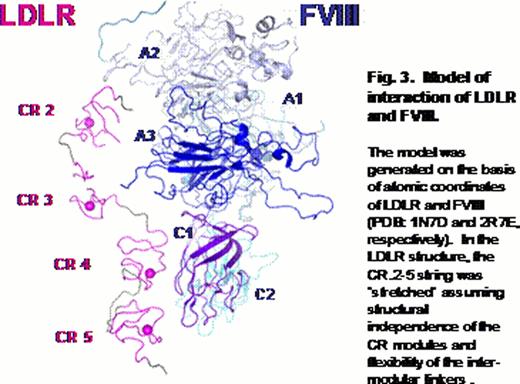
The binding site of LDLR for FVIII is formed by a region spanned by the complement-type repeats from second to fifth. We proposed a tentative model of the interaction (Fig. 3), in which this region of the receptor contacts the A3 and C1 domains of FVIII. These portions of FVIII were previously shown to be involved in interaction with another FVIII clearance receptor known as LDLR-related protein (Bovenschen N. et al, 2003 and Meems H. et al, 2011).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal