Abstract
Abstract 2193
In both canine and human patients with Immune Thrombocytopenia (ITP), bleeding risk is challenging to predict, and potentially leads to over-treatment of patients at low risk. Conversely, recent studies have highlighted the risk of thrombosis in ITP during platelet recovery. Given these clinical observations, we hypothesized that in ITP, changes in platelet response to agonists may occur in addition to changes in platelet numbers. In response to dual agonist activation (thrombin and convulxin), a subpopulation of platelets in both humans and dogs develops enhanced procoagulant activity. This subpopulation is termed coated platelets, and differences in individuals' potential to form coated platelets have been correlated with both hemorrhagic and thrombotic outcomes. In this exploratory study, we serially evaluated ex vivo platelet responsiveness to both thrombin and dual agonists (termed coated platelet potential) in a novel canine model of ITP.
Dogs (n=4) were infused with a murine monoclonal anti-GPIIb antibody (2F9) in order to model ITP and generate predictable severe thrombocytopenia. Control dogs (n=3) were infused with a control antibody. Platelet count, thrombin responsiveness, and coated platelet potential were measured at baseline, time zero, 6 hours, 24 hours, and every 24hrs thereafter until the platelet count was ≥ baseline for at least two consecutive measures (recovery). Time zero was defined as the time when platelet count first fell to ≤ 30,000/μl following 2F9 infusion, or 1 hour following control antibody infusion. For platelet thrombin responsiveness, a monoclonal antibody to P-selectin was used to determine platelet P-selectin surface expression by flow cytometry after stimulation with graded doses of thrombin. The ED50 Thrombin was defined as the concentration of thrombin required for half-maximal P-selectin expression. Coated platelet potential was defined as the percent of platelets activated to the highly procoagulant state after dual stimulation with thrombin and convulxin, as determined by binding of biotinylated fibrinogen by platelets by flow cytometry.
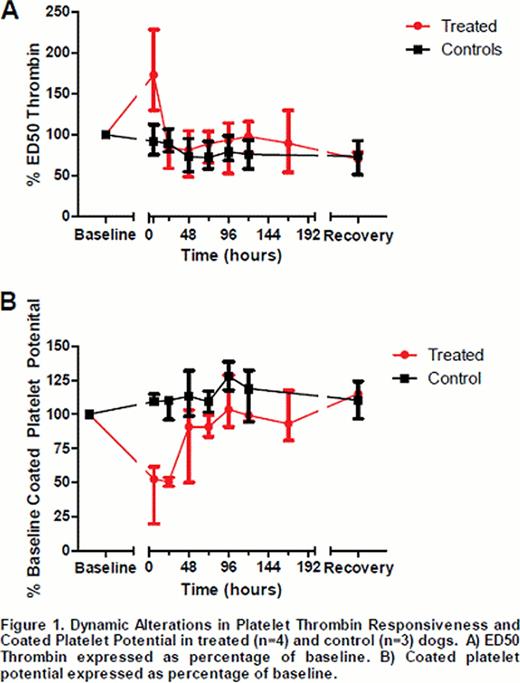
All dogs in the treated group developed severe thrombocytopenia (median=6×103, range=4–11×103 platelets/uL); no dogs in the control group developed thrombocytopenia. All treated dogs had platelet recovery by 240 hours (median=132 hours, range 120–240hours). Of interest, at 6 hours, ED50 Thrombin in the treated group increased nearly twofold (fig 1A) (ratio of median ED50 Thrombin treated/baseline=1.6, range 1.3–2.3), which correlated with a decline in coated platelet potential by nearly half of baseline (fig 1B) (median 52.4% of baseline, range 19.6–61.5%); minimal change from baseline was observed in controls. In both groups, ED50 Thrombin was lower at recovery than baseline (fig 1A) (treated median ED50 Thrombin=71.5% of baseline; control median ED50 Thrombin=67% of baseline). A trend of rising coated platelet potential was also noted as platelets recovered in the treated group.
In conclusion, in this exploratory study of a canine model of ITP, we observed dynamic changes in platelet responsiveness. During severe thrombocytopenia, we observed a rise in ED50, indicating a decline in response to thrombin, which correlated with a fall in coated platelet potential. We speculate that this early fall in platelet thrombin response and coated platelet potential could contribute to hemorrhage risk in ITP. As a complement to this finding, in the treated group, there was a rise in coated platelet potential as platelets rebounded and coated platelet potential was slightly greater than baseline at recovery. This is consistent with others' observation that younger platelets are more likely to have coated platelet potential. We also observed a decline in ED50 Thrombin at recovery, not only in the treated dogs, but also control dogs. Thus, at recovery, the decline in ED50 Thrombin was independent of treatment group. However, this may be an artifact of our small sample size. Our observed increase in coated platelet potential during platelet recovery could potentially contribute to the thrombotic tendency of some ITP patients. Future studies are planned to explore the relationship of hemorrhagic and thrombotic risk with platelet thrombin responsiveness and coated platelet potential in this model of ITP and clinical studies of canine and human ITP.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal