Abstract
Abstract 2166
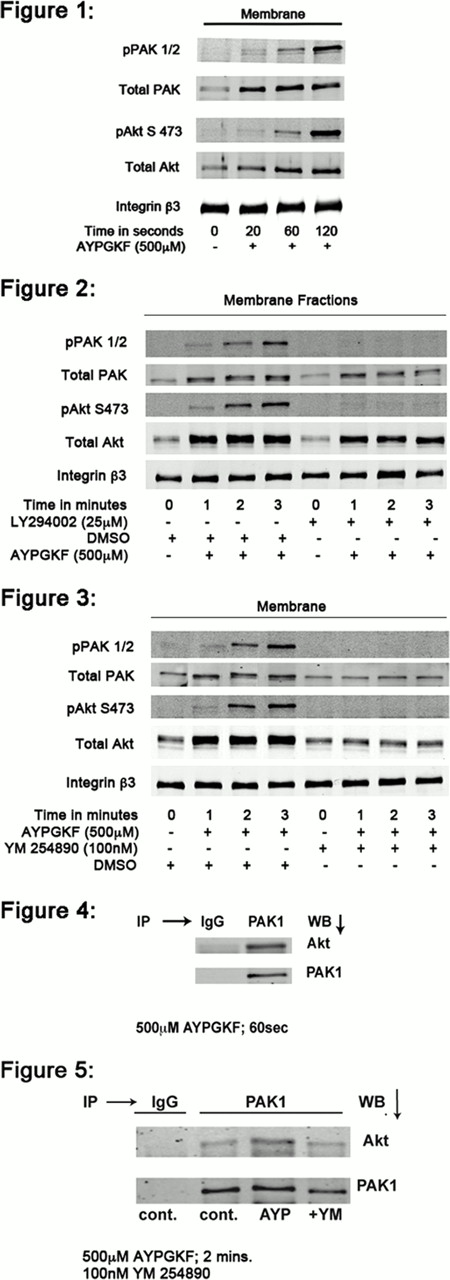
Akt has been shown to be an important signaling molecule regulating platelet aggregation. It was proposed that Akt translocates to the membrane for its activation where it is phosphorylated on Thr308 by PDK1 and Ser473 by mTORC2. However, the specific signaling events that regulate the Akt phosphorylation and its mechanism of translocation to the membrane are not clearly defined. It has been shown that Akt phosphorylation by many agonists requires signaling events downstream of Gi activation, involving PI3-kinaseb. In addition, it was proposed that Akt translocates to the plasma membrane by binding to PIP3 (Phosphatidylinositol-3,4,5-trisphosphate) generated by PI3-kinase. Our previous studies have revealed that Gq and G12/13 pathways enhance Akt phosphorylation in platelets, suggesting a role for these pathways independently of PIP3. Hence we investigated the mechanisms of Akt translocation in platelets. Stimulation of platelets with the PAR4 activation peptide (AYPGKF) caused translocation of Akt to the membrane rapidly (as early as 20 seconds) whereas, the phosphorylation of Akt occurred at the later time points (Figure 1). Pan-phoshatidylinositide 3-kinase (PI3-Kinase) inhibitors, LY-294002 (Figure 2) and Wortmannin inhibited Ser473 phosphorylation but failed to affect translocation of Akt to the membrane indicating that Akt translocation can occur independent of PI3-kinase activity. However, translocation of Akt was dramatically inhibited in the presence of a Gq-selective inhibitor, YM254890, indicating that Akt translocation is regulated downstream of Gqsignaling pathways (Figure 3).
The p21-activated kinases (PAKs) are a family of serine/threonine kinases and are key regulators of actin polymerization and cell migration. PAK functions as a scaffolding protein and is reported to interact with numerous proteins including Akt. Here, we evaluated whether PAK activated by the Gqpathways plays a significant role in the translocation of Akt to the membrane. The co-immunoprecipitation studies revealed that Akt associates with PAK upon stimulation with AYPGKF (Figure 4). This association was abolished when platelets were pre-treated with YM254890 (Figure 5).
These results suggest that PAK1/2 activation downstream of Gq pathway mediates Akt translocation to the membrane when stimulated by PAR agonist in platelets and that this translocation is PIP3- independent.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal