Abstract
Although oral iron therapy is often the initial approach to the treatment of iron deficiency anemia (IDA), many patients fail to adequately respond or do not tolerate oral iron. Unfortunately for these patients, approved treatment options are limited in the US and Canada to only the IV iron dextrans, which have boxed safety warnings and inconvenient dosing regimens. Many of these patients, therefore, do not get IV iron, and remain inadequately treated and symptomatic. Ferumoxytol (FER), a new IV iron approved for the treatment of IDA in patients with chronic kidney disease (CKD), is being investigated to treat IDA patients without CKD who have a history of unsatisfactory oral iron therapy or in whom oral iron cannot be used. This randomized, placebo-controlled, double blind trial was designed to assess the efficacy and safety of FER for the treatment of these IDA patients.
Key inclusion criteria included a Baseline hemoglobin (Hgb) less than 10 g/dL and >7 g/dL, and transferrin saturation (TSAT) <20%. Subjects were randomized 3:1 to receive 2 injections of either FER (510 mg, 5±3 days apart) or placebo (IV normal saline). Efficacy assessments included comparisons of the change in Hgb, TSAT and Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue) score in the 2 treatment groups between Baseline and Week 5.
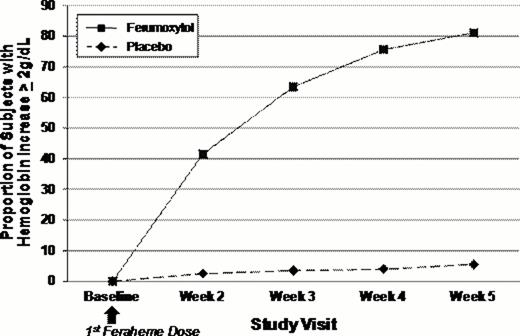
A total of 808 subjects were randomized to the 2 treatment arms (FER, n=608; placebo, n=200). FER demonstrated superiority to placebo with 81.1% of subjects achieving an increase in Hgb of >2.0 g/dL from Baseline to Week 5 compared to only 5.5% in the placebo group (treatment difference: 75.6%, p<0.0001). At each post-FER treatment time point, a larger percentage of FER-treated subjects had a >2.0 g/dL increase in Hgb compared with those treated with placebo. The superiority of FER was also demonstrated for the mean change in Hgb from Baseline to Week 5 with a robust 2.7 g/dL increase compared to only 0.1 g/dL in the placebo group (treatment difference: 2.54 g/dL, p<0.0001). An increase in TSAT from Baseline to Week 5 was only observed in FER-treated subjects (mean change: FER, 11.0%; placebo −0.1%). In addition, a statistically significant improvement in fatigue from Baseline to Week 5, as measured by the FACIT-Fatigue, was shown for FER-treated subjects compared to placebo (p<0.0001).
The rates of adverse events (AEs) and related AEs were higher in the FER group, although no pattern or safety trend was observed to suggest a specific safety signal. The overall rate of serious adverse events (SAEs) was comparable between the 2 treatment groups (FER, 2.6%; placebo, 3.0%), and treatment-related SAEs associated with the class of IV iron products were reported in 4 (0.7%) FER-treated subjects. As expected, protocol-defined AEs of Special Interest (signs/symptoms of hypotension or hypersensitivity associated with IV iron use) were noted at a higher rate in FER-treated subjects (FER, 3.6%; placebo, 1.0%). All cardiovascular AEs were considered unrelated by the investigators. Overall, FER was well tolerated and no new safety signals were identified.
Proportion of Subjects with > 2 g/dL Increase in Hgb at Any Time from Baseline to Week 5 (ITT)
Proportion of Subjects with > 2 g/dL Increase in Hgb at Any Time from Baseline to Week 5 (ITT)
Summary of Efficacy and Safety Results by Treatment Group
| . | FER (n=608) . | Placebo (n=200) . | p-value . |
|---|---|---|---|
| Efficacy Results: (ITT Population) . | |||
| % Subjects with Increase Hgb >2 g/dL1 | 81.1 | 5.5 | <0.0001 |
| Mean Change in Hgb2 (g/dL) | 2.7 | 0.1 | <0.0001 |
| Mean Change in TSAT2 (%) | 11.0 | −0.1 | <0.0001 |
| Safety Results: (Safety Population) | |||
| All Treatment Emergent AEs | 49.2% | 43.0% | |
| Related AEs | 14.6% | 7.5% | |
| All SAEs | 2.6% | 3.0% | |
| Related SAEs | 0.7% | 0% | |
| AEs of Special Interest3 | 3.6% | 1.0% | |
| Cardiovascular AEs | 0.8% | 0% | |
| AEs Resulting in Study Discontinuation | 0.5% | 1.0% | |
| . | FER (n=608) . | Placebo (n=200) . | p-value . |
|---|---|---|---|
| Efficacy Results: (ITT Population) . | |||
| % Subjects with Increase Hgb >2 g/dL1 | 81.1 | 5.5 | <0.0001 |
| Mean Change in Hgb2 (g/dL) | 2.7 | 0.1 | <0.0001 |
| Mean Change in TSAT2 (%) | 11.0 | −0.1 | <0.0001 |
| Safety Results: (Safety Population) | |||
| All Treatment Emergent AEs | 49.2% | 43.0% | |
| Related AEs | 14.6% | 7.5% | |
| All SAEs | 2.6% | 3.0% | |
| Related SAEs | 0.7% | 0% | |
| AEs of Special Interest3 | 3.6% | 1.0% | |
| Cardiovascular AEs | 0.8% | 0% | |
| AEs Resulting in Study Discontinuation | 0.5% | 1.0% | |
at any time from Baseline to Week 5.
Least Square Mean change from Baseline to Week 5.
includes protocol-defined signs/symptoms of hypotension and hypersensitivity.
In this randomized, placebo-controlled Phase III trial, 2 doses of FER were shown to be highly effective in raising hemoglobin and iron parameters in non-CKD patients with IDA who had a history of unsatisfactory oral iron therapy. FER also significantly reduced fatigue, and was generally well tolerated with no new safety signals being identified. Therefore, FER could provide an important, new treatment option for IDA patients with a history of unsatisfactory oral iron therapy or in whom oral iron could not be used.
Vadhan Raj:AMAG Pharmaceuticals, Inc.: Research Funding. Off Label Use: Feraheme (ferumoxytol) injection. For treatment of iron deficiency anemia in non-CKD patients. Cressman:AMAG Pharmaceuticals, Inc.: Employment. Strauss:AMAG Pharmaceuticals, Inc.: Employment. Bernard:AMAG Pharmaceuticals, Inc.: Employment. Li:AMAG Pharmaceuticals, Inc.: Employment. Allen:AMAG Pharmaceuticals, Inc.: Employment.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal