Abstract
Abstract 2059
Two randomized trials have demonstrated a benefit in progression free survival (PFS) of 18–20 mofor maintenance treatment with lenalidomide after auto-PBSCT for multiple myeloma (MM). Although results on survival benefit are conflicting and quality of life (QOL) data is lacking, some have begun to recommend maintenance. No study has evaluated patient perceptions of maintenance. We conducted a systematic survey of MM patients (pts) regarding what constitutes a meaningful benefit that would make burdens of maintenance (toxicity and cost) acceptable.
We mailed a brief self-administered survey in 3 waves to 1159 consecutive living pts evaluated at Mayo Clinic. The survey provided background on the standard of care for MM in transplant eligible pts and data on maintenance. Pts were asked to estimate the magnitude of OS benefit that would be acceptable for varying degrees of toxicity and cost.
Of the 1159 surveys sent, 886 pts (83.2%) responded, including 150 notifying us of a decision not to participate. 736 pts returned a completed survey (66% raw response rate). (56%) male, 407 (55%) had undergone auto-PBSCT, 467 (63%) had discussed maintenance with a physician, and 9 (1%) pts had received no treatment. 10% of pts had MM for 3–12 mo, 14% for 1–2 yr, 13% for 2–3 yr, 20% for 3–5 yr, 44% for 5 yr or more. Pt age: 4% 18–49 yr, 24% 50–59 yr, 40% 60–69 yr, 27% 70–79 yr, and 6% >80 yrs.
The most worrisome potential toxicity was identified as peripheral neuropathy by 27% of pts, cytopenias by 24%, LE DVT 20%, fatigue 15%, nausea 8%, diarrhea/constipation 7%. In response to a question about whether they would choose maintenance if there was a PFS benefit but no improvement in OS, 92% would opt for maintenance if it were known to cause mild toxicity, while 77% would opt for maintenance despite moderate toxicity.
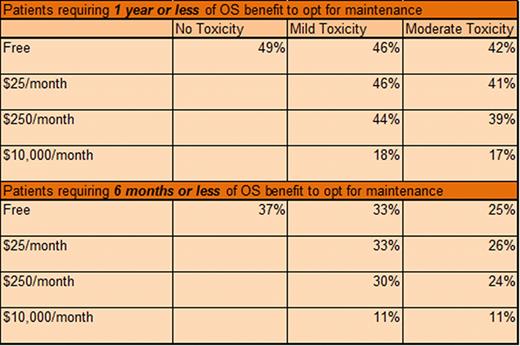
Subsequent questions included cost and toxicity considerations and required pts to identify minimum acceptable duration of OS benefit to estimate more precisely a meaningful clinical benefit. If treatment was free, had no toxicity, and the OS benefit was 1 yr or less, 49% would choose maintenance (see Figure). In the same scenario, if toxicities were mild, the proportion of pts who choose maintenance was similar (46%). If pts experienced moderate toxicity, 42% choose maintenance. With rising financial burden (out-of-pocket) and toxicity, the proportion of pts who choose maintenance declined (see Table). For example, if the cost was $25/mo, mild toxicity experienced, and the OS benefit was one year or less, 46% would choose maintenance, while 39% of pts would choose maintenance if it cost $250/month and there was moderate toxicity. If maintenance cost $10,000/mo and was associated with mild or moderate toxicity, 18% and 17% of pts would choose treatment, respectively.
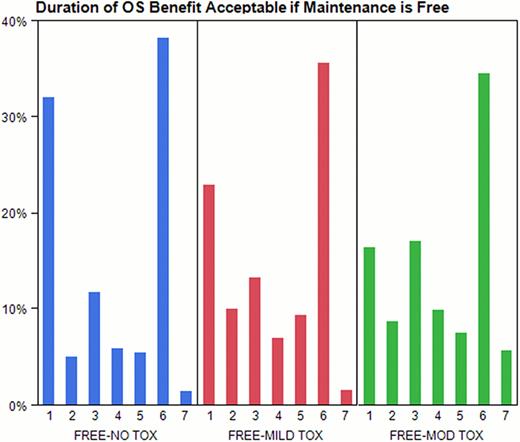
For the first time, we have described the broad range of patient perspectives regarding trade-offs in the treatment of MM with maintenance therapy. We found that willingness to be treated with maintenance declined when actual benefits were provided in concrete numeric terms compared with a general statement of PFS benefit. The discrepancy between the 92% of pts choosing to undergo maintenance if free and mild toxicity when there is a PFS benefit but no OS benefit, compared to 49% of pts choosing maintenance if free, mild toxicity, and associated with an OS benefit of 1 yror less; suggests pts have difficulty understanding the concept of PFS.
We also found that the magnitude of benefit required to consider maintenance was affected by cost and toxicity. In our experience, pts typically incur a cost of approximately $25/mo and experience mild toxicity. In this study, nearly half (46%) of such pts would opt for maintenance if the OS benefit was 1 year or less while only 33% of pts would opt for maintenance if the OS benefit was 6 months or less. Our data implies that good care in MM requires detailed conversations about each and every patient's priorities and expectations prior to making recommendations for or against such treatments.
Minimum increase in overall survival acceptable for patients with no, mild, or moderate toxicity; 1 = 3 months, 2 = 6 months, 3 = 1 year, 4 = 2 years, 5 = 3 years, 6 = 5 years, 7 = I would not choose treatment no matter the benefit.
Minimum increase in overall survival acceptable for patients with no, mild, or moderate toxicity; 1 = 3 months, 2 = 6 months, 3 = 1 year, 4 = 2 years, 5 = 3 years, 6 = 5 years, 7 = I would not choose treatment no matter the benefit.
Percent of patients finding maintenance therapy acceptable if the benefit in OS is 1 year or less versus 6 months or less for a given cost and toxicity.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal